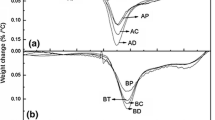
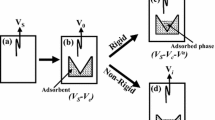
Abstract
It is shown that metastable state of the carbon matter and variability of properties associated with it are a consequence of the adaptive ability of carbon atoms to change the degree of hybridization depending on external thermodynamic conditions. Mechanisms of evolution of the coal structural and functional characteristics during interphase interaction in the coal-gas system are visualized by a qualitative assessment of the change in the distribution of carbon atoms between the sp\({}^{3}\)–sp\({}^{2}\) states. A stepwise adsorption scheme is proposed, starting from the macroscale and ending with the transformation of methane molecules and fragments of the coal aromatic structures. The results of the theoretical methods used for determining the evolution of the structure and, along with it, the reactivity of the system components in the conditions of changing characteristics of external factors confirm the substantial possibility and efficiency of the quantum-chemical modeling for identifying the peculiarities of the course of interphase interaction. The obtained results are consistent with the data of molecular spectroscopy, and the generalization of the information set is decisive for determining the driving forces of adsorption under the conditions of mechanical loading. A comprehensive approach to the systematic research allows obtaining fundamental data on the patterns of transformations of organic compounds and formation of their properties and will serve as a basis for the formation of a strategy for new-generation nanosynthesis processes and development of mechanochemistry.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Alexeev AD (2012) Physics of coal and mining processes. CRC Press, Boca Raton
Benson SW (1976) Thermochemical kinetics. John Wiley & Sons, New York-London-Sydney-Toronto
Blumenfeld LA, Voevodsky VV, Semenov AG (1962) The use of epr in chemistry. Publishing House of SO AN USSR, Novosibirsk [in Russian]
Bulat AF, Skipochka SI, Palamarchuk TA, Antsiferov VA (2010) Methangeneration in coal layers. Lyra LTD, Dnepropetrovsk (in Russian)
Bulat AF, Bogdanov VL, Trachevsky VV, Burchak OV, Serikov YuA (2021) Research of mechanisms and driving forces of the self-organization of the matrices of natural solid hidrocarbons. Dopov Nac Akad Nauk Ukr 3:26–32 [in Ukrainian]
Burchak AV, Balalaev AK (2010) The effect of changing the parameters of the IR spectra of coals in the series of metamorphism under mechanical pressure. Geotechnical mechanics: Interdepartmental collection of scientific works 87:190–198 [in Russian]
Burchak OV, Trachevsky VV, Balalaev OK (2016) The coal aromaticity coefficient comparison during carbonization. Geotechnical mechanics: Interdepartmental collection of scientific works 124:208–215 [in Ukrainian]
Butyirskaya EV (2011) Computational chemistry: bases of theory and work with the programs of Gaussian and GaussView. Solon-Press, Moscow (in Russian)
Hill J, Platts JA, Werner H-J (2006) Calculation of intermolecular interactions in the benzene dimer using coupled-cluster and local electron correlation methods. Chem Phys Phys Chem
Kolandaivel P, Nirmala V (2004) Study of proper and improper hydrogen bonding using Bader’s atoms in molecules (AIM) theory and NBO analysis. J Mol Struct
Magdesieva TV (2013) New types of combined pericyclic reactions. Russ Chem Rev 82:228–247 (in Russian)
Merrick JP, Moran D, Radom L (2007) An evaluation of harmonic vibrational frequency scale factors. J Phys Chem A
Munshi P, Guru Row TN (2005) Charge density based classification of intermolecular interactions in molecular crystals. CrystEngComm
Sordo JA (2001) On the use of the Boys-Bernardi function counterpoise procedure to correct barrier heights for basis set superposition error. J Mol Struct
Tokar A, Chigvintseva O (2021) The quantum-chemical and spectral criteria for hydrogen bonding efficiency in structural analysis of aramides. Chem Chem Technol
Tokar A, Kabat O, Chigvintseva O, Belošević S (2021) Intermolecular interactions in complex systems “Polyamide-Silica Gel”: The quantum-chemical interpretation. In: Karabegović I (eds) New technologies, development and application IV. Lecture Notes in Networks and Systems, vol. 233. Springer, Cham, pp. 875–882
Tsirelson VG (2017) The quantum chemistry. Molecules, molecular systems and solids. Laboratory of Knowledge, Moscow [in Russian]
Weinhold F (2012) Natural bond orbital analysis: A critical overview of relationships to alternative bonding perspectives. J Comput Chem
Weinhold F, Landis C (2012) Discovering chemistry with natural bond orbitals. Wiley, New Jersey
Zhikol O, Shishkin OV, Lyssenko KA, Leszczynski J (2005) Electron density distribution in stacked benzene dimers: A new approach towards the estimation of stacking interaction energies. J Chem Phys
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bulat, A., Burchak, O., Trachevskyi, V., Tokar, A. (2023). Evolution of Electron Structure of the Methane-Coal Sorption System Components and Properties. In: Guz, A.N., Altenbach, H., Bogdanov, V., Nazarenko, V.M. (eds) Advances in Mechanics. Advanced Structured Materials, vol 191. Springer, Cham. https://doi.org/10.1007/978-3-031-37313-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-37313-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-37312-1
Online ISBN: 978-3-031-37313-8
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)