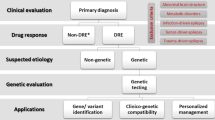
Abstract
This chapter is devoted to drug-resistant epilepsy and its genetic mechanisms. There are currently six hypotheses proposed for pharmacoresistant epilepsy. The genetic aspects of five of the six hypotheses are addressed in this chapter except from the intrinsic hypothesis as it does not comprise genetic mechanisms.
In the end, we propose two assertions in the definition of genetic “drug-resistant” epilepsies: (1) when the antiseizure medication (ASM) has a proven effect on the molecular lesion, but seizures persist in spite of ASM treatment; this latter, we believe is true genetic pharmacorresistant epilepsy and (2) epilepsies are drug resistant because the antiseizure drug ASM does not have an effect on the specific molecular lesion of the epilepsy syndrome. The epilepsy is supposedly “drug resistant,” but seizures do not stop because “the key does not fit the lock in the door”.
In diagnosis poor response to treatment, it is also important to consider that incorrect diagnosis in some epilepsies can also lead to “pseudo-drug resistance,” where mistreatment can lead to poor response or aggravation of seizures, this happens more frequently in genetic epilepsies.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Al-Eitan LN, Al-Dalalah IM, Mustafa MM, Alghamdi MA, Elshammari AK, Khreisat WH, Aljamal HA. Effects of MTHFR and ABCC2 gene polymorphisms on antiepileptic drug responsiveness in Jordanian epileptic patients. Pharmacogenom Personal Med. 2019;12:87–95. https://doi.org/10.2147/PGPM.S211490.
Angelman H. Puppet children: a report on three cases. Dev Med Child Neurol 1965;7:681–8.
Balan S, Sathyan S, Radha SK, Joseph V, Radhakrishnan K, Banerjee M. GABRG2, rs211037 is associated with epilepsy susceptibility, but not with antiepileptic drug resistance and febrile seizures. Pharmacogenet Genomics. 2013;23(11):605–10. https://doi.org/10.1097/FPC.0000000000000000.
Brandolese R, Scordo MG, Spina E, Gusella M, Padrini R. Severe phenytoin intoxication in a subject homozygous for CYP2C9*3. Clin Pharmacol Therapeutics. 2001;70(4):391–4. https://doi.org/10.1016/S0009-9236(01)95478-5.
Cassidy SB, Thuline HC, Holm VA. Deletion of chromosome 15 (q11-13) in a Prader-Labhart-Willi syndrome clinic population. Am J Med Genet. 1984;17(2):485–95.
Chan EM, Young EJ, Ianzano L, Munteanu I, Zhao X, Christopoulos CC, et al. Mutations in NHLRC1 cause progressive myoclonus epilepsy. Nat Genet. 2003;35(2):125–7.
Canevini MP, Sgro V, Zuffardi O, Canger R, Carrozzo R, Rossi E, et al. Chromosome 20 ring: a chromosomal disorder associated with a particular electroclinical pattern. Epilepsia. 1998;39(9):942–51.
Cascorbi I, Gerloff T, Johne A, Meisel C, Hoffmeyer S, Schwab M, et al. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Therapeutics. 2001;69(3):169–74. https://doi.org/10.1067/MCP.2001.114164.
Chen J, Su Q, Qin J, Zhou Y, Ruan H, Chen Z, et al. Correlation of MCT1 and ABCC2 gene polymorphisms with valproic acid resistance in patients with epilepsy on valproic acid monotherapy. Drug Metab Pharmacokinet. 2019;34(3):165–71. https://doi.org/10.1016/J.DMPK.2018.01.006.
Chioza B, Wilkie H, Nashef L, Blower J, McCormick D, Sham P, Asherson P, Makoff AJ. Association between the alpha(1a) calcium channel gene CACNA1A and idiopathic generalized epilepsy. Neurology. 2001;56(9):1245–6.
Czornyj L, Auzmendi J, Lazarowski A. Transporter hypothesis in pharmacoresistant epilepsies. Is it at the central or peripheral level? Epilepsia Open. 2022;7 Suppl 1(Suppl 1):S34–S46. https://doi.org/10.1002/EPI4.12537.
Deeken JF, Löscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13(6):1663–74.
Dobyns WB, Reiner O, Carrozzo R, Ledbetter DH. Lissencephaly. A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. JAMA. 1993;270(23):2838–42.
Dravet C. - Vie Med. Les epilepsies graves de l’enfant. 1978;8:543–48.
Fang M, ** ZQ, Wu Y, Wang XF. A new hypothesis of drug refractory epilepsy: neural network hypothesis. Med Hypotheses. 2011;76(6):871–6.
Feng W, Mei S, Zhu L, Yu Y, Yang W, Gao B, et al. Effects of UGT2B7, SCN1A and CYP3A4 on the therapeutic response of sodium valproate treatment in children with generalized seizures. Seizure. 2018;58:96–100. https://doi.org/10.1016/J.SEIZURE.2018.04.006.
Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67(6):1047–58.
Gambardella A, Manna I, Labate A, Chifari R, la Russa A, Serra P, et al. GABA(B) receptor 1 polymorphism (G1465A) is associated with temporal lobe epilepsy. Neurology. 2003;60(4):560–3. https://doi.org/10.1212/01.WNL.0000046520.79877.D8.
Ganesh S, Delgado-Escueta AV, Sakamoto T, Avila MR, Machado-Salas J, Hoshii Y, et al. Targeted disruption of the Epm2a gene causes formation of Lafora inclusion bodies, neurodegeneration, ataxia, myoclonus epilepsy and impaired behavioral response in mice. Hum Mol Genet. 2002a;11(11):1251–62.
Ganesh S, Delgado-Escueta AV, Suzuki T, Francheschetti S, Riggio C, Avanzini G, et al. Genotype-phenotype correlations for EPM2A mutations in Lafora’s progressive myoclonus epilepsy: exon 1 mutations associate with an early-onset cognitive deficit subphenotype. Hum Mol Genet. 2002b;11(11):1263–71.
Gao L, Yin X, Li Y, **ao H, Yang L, Fan H, et al. Association of MDR1 gene polymorphisms with refractory epilepsy in children. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2019;36(11):1073–6. https://doi.org/10.3760/CMA.J.ISSN.1003-9406.2019.11.004.
Ghosh C, Gonzalez-Martinez J, Hossain M, Cucullo L, Fazio V, Janigro D, Marchi N. Pattern of P450 expression at the human blood-brain barrier: roles of epileptic condition and laminar flow. Epilepsia. 2010;51(8):1408–17. https://doi.org/10.1111/J.1528-1167.2009.02428.X.
Ghosh C, Marchi N, Desai NK, Puvenna V, Hossain M, Gonzalez-Martinez J, et al. Cellular localization and functional significance of CYP3A4 in the human epileptic brain. Epilepsia. 2011;52(3):562–71. https://doi.org/10.1111/J.1528-1167.2010.02956.X.
Gogou M, Pavlou E. Efficacy of antiepileptic drugs in the era of pharmacogenomics: a focus on childhood. Eur J Paediatr Neurol. 2019;23:674–84. W.B. Saunders Ltd. https://doi.org/10.1016/j.ejpn.2019.06.004.
Gonzalez-Giraldo E, Sullivan JE. Advances in the treatment of drug-resistant pediatric Epilepsy. Semin Neurol. 2020;40(2):257–62. https://doi.org/10.1055/s-0040-1702941.
Harkin LA, McMahon JM, Iona X, Dibbens L, Pelekanos JT, Zuberi SM, et al. Infantile Epileptic Encephalopathy Referral Consortium, Sutherland G, Berkovic SF, Mulley JC, Scheffer IE. The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain. 2007;130(Pt 3):843–52.
Hauser RM, Henshall DC, Lubin FD. The epigenetics of epilepsy and its progression. Neuroscientist. 2018;24(2):186–200.
Juvale IIA, Che Has AT. Possible interplay between the theories of pharmacoresistant epilepsy. Eur J Neurosci. 2021;53(6):1998–2026. https://doi.org/10.1111/EJN.15079.
Klepper J, Voit T. Facilitated glucose transporter protein type 1 (GLUT1) deficiency syndrome: impaired glucose transport into brain– a review. Eur J Pediatr. 2002;161(6):295–304.
Kong FC, Ma CL, Lang LQ, Zhong MK. Association of xenobiotic receptor polymorphisms with carbamazepine response in epilepsy patients. Gene. 2021;771 https://doi.org/10.1016/J.GENE.2020.145359.
Kubota H, Ishihara H, Langmann T, Schmitz G, Stieger B, Wieser HG, et al. Distribution and functional activity of P-glycoprotein and multidrug resistance associated proteins in human brain microvascular endothelial cells in hippocampal sclerosis. Epilepsy Res. 2006;68(3):213–28.
Lancelin F, Franchon E, Kraoul L, Garciau I, Brovedani S, Tabaouti K, et al. Therapeutic drug monitoring of levetiracetam by high-performance liquid chromatography with photodiode array ultraviolet detection: preliminary observations on correlation between plasma concentration and clinical response in patients with refractory epilepsy. Ther Drug Monit. 2007;29(5):576–83. https://doi.org/10.1097/FTD.0B013E318157032D.
Lazarowski A, Czornyj L. Potential role of multidrug resistant proteins in refractory epilepsy and antiepileptic drugs interactions. Drug Metabol Drug Interact. 2011;26(1):21–6. https://doi.org/10.1515/DMDI.2011.006/MACHINEREADABLECITATION/RIS.
Lazarowski A, Sevlever G, Taratuto A, Massaro M, Rabinowicz A. Tuberous sclerosis associated with MDR1 gene expression and drug- resistant epilepsy. Pediatr Neurol. 1999;21(4):731–4. https://doi.org/10.1016/S0887-8994(99)00074-0.
Leandro K, Bicker J, Alves G, Falcão A, Fortuna A. ABC transporters in drug-resistant epilepsy: mechanisms of upregulation and therapeutic approaches. Pharmacol Res. 2019;144:357–76. https://doi.org/10.1016/J.PHRS.2019.04.031.
López-García MA, Feria-Romero IA, Serrano H, Rayo-Mares D, Fagiolino P, Vázquez M, et al. Influence of genetic variants of CYP2D6, CYP2C9, CYP2C19 and CYP3A4 on antiepileptic drug metabolism in pediatric patients with refractory epilepsy. Pharmacol Rep. 2017;69(3):504–11. https://doi.org/10.1016/J.PHAREP.2017.01.007.
Löscher W, Poulter MO, Padjen AL. Major targets and mechanisms of antiepileptic drugs and major reasons for failure. Adv Neurol. 2006;97:417–27.
Löscher W, Potschka H, Sisodiya SM, Vezzani A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol Rev. 2020;72(3):606–38.
Łukawski K, Czuczwar SJ. Understanding mechanisms of drug resistance in epilepsy and strategies for overcoming it. Expert Opin Drug Metab Toxicol. 2021;17(9):1075–90. https://doi.org/10.1080/17425255.2021.1959912.
Ma S, Abou-Khalil B, Sutcliffe JS, Haines JL, Hedera P. The GABBR1 locus and the G1465A variant is not associated with temporal lobe epilepsy preceded by febrile seizures. BMC Med Genet. 2005;6(1):1–5. https://doi.org/10.1186/1471-2350-6-13/PEER-REVIEW.
Makowska M, Smolarz B, Bryś M, Forma E, Romanowicz H. An association between the rs1799853 and rs1057910 polymorphisms of CYP2C9, the rs4244285 polymorphism of CYP2C19 and the prevalence rates of drug-resistant epilepsy in children. 2020;131(12):1147–54. https://doi.org/10.1080/00207454.2020.1781110.
Miller JQ- Lissencephaly in 2 siblings. Neurology. 1963;13:841–50.
Miller DS, Bauer B, Hartz AMS. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60(2):196–209. https://doi.org/10.1124/PR.107.07109.
Minassian BA, DeLorey TM, Olsen RW, Philippart M, Bronstein Y, Zhang Q, et al. Angelman syndrome: correlations between epilepsy phenotypes and genotypes. Ann Neurol. 1998;43(4):485–93.
Mohammed Ebid AHI, Ahmed MMM, Mohammed SA. Therapeutic drug monitoring and clinical outcomes in epileptic Egyptian patients: a gene polymorphism perspective study. Ther Drug Monit. 2007;29(3):305–12. https://doi.org/10.1097/FTD.0B013E318067CE90.
Mosyagin I, Runge U, Schroeder HW, Dazert E, Vogelgesang S, Siegmund W, et al. Association of ABCB1 genetic variants 3435C>T and 2677G>T to ABCB1 mRNA and protein expression in brain tissue from refractory epilepsy patients. Epilepsia. 2008;49(9):1555–61. https://doi.org/10.1111/J.1528-1167.2008.01661.X.
Ohmori I, Ouchida M, Ohtsuka Y, Oka E, Shimizu K. Significant correlation of the SCN1A mutations and severe myoclonic epilepsy in infancy. Biochem Biophys Res Commun. 2002;295(1):17–23.
Pérez-Pérez D, Frías-Soria CL, Rocha L. Drug-resistant epilepsy: from multiple hypotheses to an integral explanation using preclinical resources. Epilepsy Behav. 2021;121(Pt B) https://doi.org/10.1016/J.YEBEH.2019.07.031.
Povey S, Burley MW, Attwood J, Benham F, Hunt D, Jeremiah SJ, et al. Two loci for tuberous sclerosis: one on 9q34 and one on 16p13. Ann Hum Genet. 1994;58(Pt 2):107–27.
Rizzi M, Caccia S, Guiso G, Richichi C, Gorter JA, Aronica E, et al. Limbic seizures induce P-glycoprotein in rodent brain: functional implications for pharmacoresistance. J Neurosci. 2002;22(14):5833–9. https://doi.org/10.1523/JNEUROSCI.22-14-05833.2002.
Saleem T, Maqbool H, Sheikh N, Tayyeb A, Mukhtar M, Ashfaq A. GABRG2 C588T polymorphism is associated with idiopathic generalized Epilepsy but not with antiepileptic drug resistance in Pakistani Cohort. Biomed Res Int. 2022;2022:3460792. https://doi.org/10.1155/2022/3460792.
Schmidt D, Löscher W. New developments in antiepileptic drug resistance: an integrative view. Epilepsy Curr. 2009;9(2):47–52.
Sears SMS, Hewett SJ. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exper Biol Med. 2021;246:1069–83. SAGE Publications Inc. https://doi.org/10.1177/1535370221989263.
Shahwan A, Farrell M, Delanty N. Progressive myoclonic epilepsies: a review of genetic and therapeutic aspects. Lancet Neurol. 2005;4(4):239–48.
Sheilabi MA, Takeshita LY, Sims EJ, Falciani F, Princivalle AP. The sodium channel B4-subunits are dysregulated in temporal lobe epilepsy drug-resistant patients. Int J Mol Sci. 2020;21(8):2955.
Shen CH, Zhang YX, Lu RY, ** B, Wang S, Liu ZR, et al. Specific OCT1 and ABCG2 polymorphisms are associated with Lamotrigine concentrations in Chinese patients with epilepsy. Epilepsy Res. 2016;127:186–90. https://doi.org/10.1016/J.EPLEPSYRES.2016.09.004.
Shin HS, Cheong EJ, Choi S, Lee J, Na HS. T-type Ca2+ channels as therapeutic targets in the nervous system. Curr Opin Pharmacol. 2008;8(1):33–41.
Shlobin NA, Sander JW. Current principles in the management of drug-resistant Epilepsy. CNS Drugs. 2022;36(6):555–68. https://doi.org/10.1007/S40263-022-00922-4.
Singh R, Andermann E, Whitehouse WP, Harvey AS, Keene DL, Seni MH, Crossland KM, Andermann F, Berkovic SF, Scheffer IE. Severe myoclonic epilepsy of infancy: extended spectrum of GEFS+? Epilepsia. 2001;42:837–44.
Siddiqui A, Kerb R, Weale ME, Brinkmann U, Smith A, Goldstein DB, et al. Association of multidrug resistance in Epilepsy with a polymorphism in the drug-transporter gene ABCB1. 2003;348(15):1442–8. https://doi.org/10.1056/NEJMOA021986.
Sills GJ, Mohanraj R, Butler E, McCrindle S, Collier L, Wilson EA, Brodie MJ. Lack of association between the C3435T polymorphism in the human multidrug resistance (MDR1) gene and Response to antiepileptic drug treatment. Epilepsia. 2005;46(5):643–7. https://doi.org/10.1111/J.1528-1167.2005.46304.X.
Sisodiya SM, Thom M. Widespread upregulation of drug-resistance proteins in fatal human status epilepticus. Epilepsia. 2003;44(2):261–4. https://doi.org/10.1046/J.1528-1157.2003.42802.X.
Skalski D, Smolarz B, Wendorff J. Zwia˛zek pomie˛dzy polimorfizmami pojedynczych nukleotydów genu opornos’ci wielolekowej typu 1. a padaczka˛ lekooporna. Neuropsychiatry Neuropsychol. 2011;2:79–84.
Smolarz B, Makowska M, Romanowicz H. Pharmacogenetics of drug-resistant epilepsy (Review of literature). Int J Mol Sci. 2021, November 1;22. MDPI. https://doi.org/10.3390/ijms222111696.
Soranzo N, Goldstein DB, Sisodiya SM. The role of common variation in drug transporter genes in refractory epilepsy. 2005;6(8):1305–12. https://doi.org/10.1517/14656566.6.8.1305.
Tamimi DE, Abduljabbar R, Yousef AM, Saeed RM, Zawiah M. Association between ABCB1 polymorphisms and response to antiepileptic drugs among Jordanian epileptic patients. Neurol Res. 2021;43(9):724–35. https://doi.org/10.1080/01616412.2021.1922182.
Tan NCK, Heron SE, Scheffer IE, Pelekanos JT, McMahon JM, Vears DF, et al. Failure to confirm association of a polymorphism in ABCB1 with multidrug-resistant epilepsy. Neurology. 2004;63(6):1090–2. https://doi.org/10.1212/01.WNL.0000137051.33486.C7.
Tang F, Hartz AMS, Bauer B. Drug-resistant Epilepsy: multiple hypotheses, few answers. Front Neurol. 2017;8(JUL) https://doi.org/10.3389/FNEUR.2017.00301.
Vahab SA, Sen S, Ravindran N, Mony S, Mathew A, Vuayan N, et al. Analysis of genotype and haplotype effects of ABCB1 (MDR1) polymorphisms in the risk of medically refractory Epilepsy in an Indian population. Drug Metab Pharmacokinet. 2009;24(3):255–60. https://doi.org/10.2133/DMPK.24.255.
van der Weide J, Steijns LSW, van Weelden MJM, de Haan K. The effect of genetic polymorphism of cytochrome P450 CYP2C9 on phenytoin dose requirement. Pharmacogenetics. 2001;11(4):287–91. https://doi.org/10.1097/00008571-200106000-00002.
van Vliet EA, Redeker S, Aronica E, Edelbroek PM, Gorter JA. Expression of multidrug transporters MRP1, MRP2, and BCRP shortly after status epilepticus, during the latent period, and in chronic epileptic rats. Epilepsia. 2005;46(10):1569–80. https://doi.org/10.1111/j.1528-1167.2005.00250.x
van Vliet EA, van Schaik R, Edelbroek PM, Voskuyl RA, Redeker S, Aronica E, et al. Region-specific overexpression of P-glycoprotein at the blood-brain barrier affects brain uptake of phenytoin in epileptic rats. J Pharmacol Exp Ther. 2007;322(1):141–7. https://doi.org/10.1124/JPET.107.121178.
van Vliet EA, Zibell G, Pekcec A, Schlichtiger J, Edelbroek PM, Holtman L, et al. COX-2 inhibition controls P-glycoprotein expression and promotes brain delivery of phenytoin in chronic epileptic rats. Neuropharmacology. 2010;58(2):404–12. https://doi.org/10.1016/J.NEUROPHARM.2009.09.012.
von Stülpnagel C, Plischke H, Zill P, Bäumel C, Spiegel R, Gruber R, Kluger G. Letter: lack of association between MDR1 polymorphisms and pharmacoresistance to anticonvulsive drugs in patients with childhood-onset epilepsy. Epilepsia. 2009;50(7):1835–7. https://doi.org/10.1111/J.1528-1167.2009.02077.X.
Yang X, Yan Y, Fang S, Zeng S, Ma H, Qian L, et al. Comparison of oxcarbazepine efficacy and MHD concentrations relative to age and BMI associations among ABCB1, ABCC2, UGT2B7, and SCN2A polymorphisms. Medicine (United States). 2019;98(12) https://doi.org/10.1097/MD.0000000000014908.
Zan X, Yue G, Hao Y, Sima X. A systematic review and meta-analysis of the association of ABCC2/ABCG2 polymorphisms with antiepileptic drug responses in epileptic patients. Epilepsy Res. 2021;175:106678. https://doi.org/10.1016/J.EPLEPSYRES.2021.106678.
Zhao T, Yu J, Wang TT, Feng J, Zhao WB, Sun L, et al. Impact of ABCB1 polymorphism on levetiracetam serum concentrations in epileptic Uygur children in China. Ther Drug Monit. 2020;42(6):886–92. https://doi.org/10.1097/FTD.0000000000000805.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Martínez-Juárez, I.E. et al. (2023). Genes Involved in Pharmacoresistant Epilepsy. In: Rocha, L.L., Lazarowski, A., Cavalheiro, E.A. (eds) Pharmacoresistance in Epilepsy. Springer, Cham. https://doi.org/10.1007/978-3-031-36526-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-031-36526-3_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-36525-6
Online ISBN: 978-3-031-36526-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)