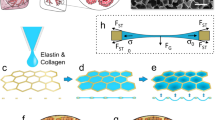
Abstract
Since the publication of the first lung-on-a-chip in 2010, research has made tremendous progress in mimicking the cellular environment of healthy and diseased alveoli. As the first lung-on-a-chip products have recently reached the market, innovative solutions to even better mimic the alveolar barrier are paving the way for the next generation lung-on-chips. The original polymeric membranes made of PDMS are being replaced by hydrogel membranes made of proteins from the lung extracellular matrix, whose chemical and physical properties exceed those of the original membranes. Other aspects of the alveolar environment are replicated, such as the size of the alveoli, their three-dimensional structure, and their arrangement. By tuning the properties of this environment, the phenotype of alveolar cells can be tuned, and the functions of the air-blood barrier can be reproduced, allowing complex biological processes to be mimicked. Lung-on-a-chip technologies also provide the possibility of obtaining biological information that was not possible with conventional in vitro systems. Pulmonary edema leaking through a damaged alveolar barrier and barrier stiffening due to excessive accumulation of extracellular matrix proteins can now be reproduced. Provided that the challenges of this young technology are overcome, there is no doubt that many application areas will benefit greatly.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–8.
Guenat OT, Geiser T, Berthiaume F. Clinically Relevant Tissue Scale Responses as New Readouts from Organs-on-a-Chip for Precision Medicine. Annu Rev Anal Chem (Palo Alto Calif). 2020;13:111–33.
Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 2012;4:159ra147.
Weibel ER, Gomez DM. Architecture of the human lung. Use of quantitative methods establishes fundamental relations between size and number of lung structures. Science. 1962;137:577–85.
Basil MC, Katzen J, Engler AE, Guo M, Herriges MJ, Kathiriya JJ, et al. The Cellular and Physiological Basis for Lung Repair and Regeneration: Past, Present, and Future. Cell Stem Cell. Elsevier Inc.; 2020;26:482–502.
Zepp JA, Morrisey EE. Cellular crosstalk in the development and regeneration of the respiratory system. Nature Reviews Molecular Cell Biology [Internet]. Springer US; 2019; Available from: https://doi.org/10.1038/s41580-019-0141-3
Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, et al. Type 2 alveolar cells are stem cells in adult lung. Journal of Clinical Investigation. 2013;123:3025–36.
Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. Nature Publishing Group; 2018;555:251–5.
Strunz M, Simon LM, Ansari M, Kathiriya JJ, Angelidis I, Mayr CH, et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nature Communications. Springer US; 2020;11:3559.
Travaglini KJ, Nabhan AN, Penland L, Sinha R, Gillich A, Sit RV, et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature. 2020;587:619–25.
Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP, et al. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell. 2017;170:1134–1148.e10.
Guenat OT, Berthiaume F. Incorporating mechanical strain in organs-on-a-chip: Lung and skin. Biomicrofluidics. 2018;12.
Gehr P, Bachofen M, Weibel ER. The normal human lung: ultrastructure and morphometric estimation of diffusion capacity. Respiration Physiology. 1978;32:121–40.
Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol. 2015;16:36–44.
Ochs M, Nyengaard JR, Jung A, Knudsen L, Voigt M, Wahlers T, et al. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169:120–4.
Waters CM, Roan E, Navajas D. Mechanobiology in lung epithelial cells: measurements, perturbations, and responses. Compr Physiol. 2012;2:1–29.
Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J. 2017;50:1601805.
Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, et al. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417–23.
Tièche CC, Gao Y, Bührer ED, Hobi N, Berezowska SA, Wyler K, et al. Tumor Initiation Capacity and Therapy Resistance Are Differential Features of EMT-Related Subpopulations in the NSCLC Cell Line A549. Neoplasia. 2019;21:185–96.
Miller AJ, Dye BR, Ferrer-Torres D, Hill DR, Overeem AW, Shea LD, et al. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc. 2019;14:518–40.
Jacob A, Morley M, Hawkins F, McCauley KB, Jean JC, Heins H, et al. Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells. Cell Stem Cell. Elsevier Inc.; 2017;21:472–488.e10.
Ghaedi M, Mendez JJ, Bove PF, Sivarapatna A, Raredon MSB, Niklason LE. Alveolar epithelial differentiation of human induced pluripotent stem cells in a rotating bioreactor. Biomaterials. 2014;35:699–710.
Tamò L, Hibaoui Y, Kallol S, Alves MP, Albrecht C, Hostettler KE, et al. Generation of an alveolar epithelial type II cell line from induced pluripotent stem cells. Am J Physiol Lung Cell Mol Physiol. 2018;315:L921–32.
Alysandratos K-D, Russo SJ, Petcherski A, Taddeo EP, Acín-Pérez R, Villacorta-Martin C, et al. Patient-specific iPSCs carrying an SFTPC mutation reveal the intrinsic alveolar epithelial dysfunction at the inception of interstitial lung disease. Cell Rep. 2021;36:109636.
Youk J, Kim T, Evans KV, Jeong Y-I, Hur Y, Hong SP, et al. Three-Dimensional Human Alveolar Stem Cell Culture Models Reveal Infection Response to SARS-CoV-2. Cell Stem Cell. Elsevier Inc.; 2020;27:905–919.e10.
Weiner AI, Jackson SR, Zhao G, Quansah KK, Farshchian JN, Neupauer KM, et al. Mesenchyme-free expansion and transplantation of adult alveolar progenitor cells: steps toward cell-based regenerative therapies. NPJ Regen Med. 2019;4:17.
Nikolić MZ, Rawlins EL. Lung Organoids and Their Use To Study Cell-Cell Interaction. Curr Pathobiol Rep. 2017;5:223–31.
Leeman KT, Pessina P, Lee J-H, Kim CF. Mesenchymal Stem Cells Increase Alveolar Differentiation in Lung Progenitor Organoid Cultures. Scientific Reports. Springer US; 2019;9:6479.
Evans KV, Lee J-H. Alveolar wars: The rise of in vitro models to understand human lung alveolar maintenance, regeneration, and disease. Stem Cells Transl Med. 2020;9:867–81.
Stucki AO, Stucki JD, Hall SRR, Felder M, Mermoud Y, Schmid RA, et al. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip. 2015;15:1302–10.
Zamprogno P, Wüthrich S, Achenbach S, Thoma G, Stucki JD, Hobi N, et al. Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun Biol. 2021;4:168.
Huang D, Liu T, Liao J, Maharjan S, **e X, Pérez M, et al. Reversed-engineered human alveolar lung-on-a-chip model. Proc Natl Acad Sci U S A. 2021;118:e2016146118.
Burgess JK, Mauad T, T** G, Karlsson JC, Westergren-Thorsson G. The extracellular matrix - the under-recognized element in lung disease? J Pathol. 2016;240:397–409.
Zhou Y, Horowitz JC, Naba A, Ambalavanan N, Atabai K, Balestrini J, et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biology [Internet]. Elsevier B.V.; 2018; Available from: https://doi.org/10.1016/j.matbio.2018.03.005
Tan X, Rodrigue D. A Review on Porous Polymeric Membrane Preparation. Part II: Production Techniques with Polyethylene, Polydimethylsiloxane, Polypropylene, Polyimide, and Polytetrafluoroethylene. Polymers (Basel). 2019;11:E1310.
van Meer BJ, de Vries H, Firth KSA, van Weerd J, Tertoolen LGJ, Karperien HBJ, et al. Small molecule absorption by PDMS in the context of drug response bioassays. Biochem Biophys Res Commun. 2017;482:323–8.
Petrou CL, D’Ovidio TJ, Bölükbas DA, Tas S, Brown RD, Allawzi A, et al. Clickable decellularized extracellular matrix as a new tool for building hybrid-hydrogels to model chronic fibrotic diseases in vitro. J Mater Chem B. 2020;8:6814–26.
Nishiguchi A, Singh S, Wessling M, Kirkpatrick CJ, Möller M. Basement Membrane Mimics of Biofunctionalized Nanofibers for a Bipolar-Cultured Human Primary Alveolar-Capillary Barrier Model. Biomacromolecules. 2017;18:719–27.
Higuita-Castro N, Nelson MT, Shukla V, Agudelo-Garcia PA, Zhang W, Duarte-Sanmiguel SM, et al. Using a Novel Microfabricated Model of the Alveolar-Capillary Barrier to Investigate the Effect of Matrix Structure on Atelectrauma. Sci Rep. 2017;7:11623.
Dohle E, Singh S, Nishigushi A, Fischer T, Wessling M, Möller M, et al. Human Co- and Triple-Culture Model of the Alveolar-Capillary Barrier on a Basement Membrane Mimic. Tissue Eng Part C Methods. 2018;24:495–503.
Yang X, Li K, Zhang X, Liu C, Guo B, Wen W, et al. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab Chip. 2018;18:486–95.
Pensabene V, Costa L, Terekhov AY, Gnecco JS, Wikswo JP, Hofmeister WH. Ultrathin Polymer Membranes with Patterned, Micrometric Pores for Organs-on-Chips. ACS Appl Mater Interfaces. 2016;8:22629–36.
Montesanto S, Smithers NP, Bucchieri F, Brucato V, La Carrubba V, Davies DE, et al. Establishment of a pulmonary epithelial barrier on biodegradable poly-L-lactic-acid membranes. PLoS One. 2019;14:e0210830.
Tas S, Rehnberg E, Bölükbas DA, Beech JP, Kazado LN, Svenningsson I, et al. 3D printed lung on a chip device with a stretchable nanofibrous membrane for modeling ventilator induced lung injury [Internet]. Bioengineering; 2021 Jul. Available from: http://biorxiv.org/lookup/doi/10.1101/2021.07.02.450873
Dunphy SE, Bratt JAJ, Akram KM, Forsyth NR, El Haj AJ. Hydrogels for lung tissue engineering: Biomechanical properties of thin collagen-elastin constructs. Journal of the Mechanical Behavior of Biomedical Materials. 2014;38:251–9.
Zamprogno P, Thoma G, Cencen V, Ferrari D, Putz B, Michler J, et al. Mechanical Properties of Soft Biological Membranes for Organ-on-a-Chip Assessed by Bulge Test and AFM. ACS Biomater Sci Eng. 2021;7:2990–7.
Barkal LJ, Procknow CL, Álvarez-García YR, Niu M, Jiménez-Torres JA, Brockman-Schneider RA, et al. Microbial volatile communication in human organotypic lung models. Nat Commun. 2017;8:1770.
Stucki JD, Hobi N, Galimov A, Stucki AO, Schneider-Daum N, Lehr C-M, et al. Medium throughput breathing human primary cell alveolus-on-chip model. Sci Rep. 2018;8:14359.
Hassell BA, Goyal G, Lee E, Sontheimer-Phelps A, Levy O, Chen CS, et al. Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro. Cell Reports. ElsevierCompany.; 2017;21:508–16.
Thacker VV, Dhar N, Sharma K, Barrile R, Karalis K, McKinney JD. A lung-on-chip model of early Mycobacterium tuberculosis infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth. Elife. 2020;9:e59961.
Seo J, Conegliano D, Farrell M, Cho M, Ding X, Seykora T, et al. A microengineered model of RBC transfusion-induced pulmonary vascular injury. Sci Rep. 2017;7:3413.
Varone A, Nguyen JK, Leng L, Barrile R, Sliz J, Lucchesi C, et al. A novel organ-chip system emulates three-dimensional architecture of the human epithelia and the mechanical forces acting on it. Biomaterials. 2021;275:120957.
Saito A, Nagase T. Hippo and TGF-β interplay in the lung field. Am J Physiol Lung Cell Mol Physiol. 2015;309:L756–767.
Rentzsch I, Santos CL, Huhle R, Ferreira JMC, Koch T, Schnabel C, et al. Variable stretch reduces the pro-inflammatory response of alveolar epithelial cells. PLoS One. 2017;12:e0182369.
Diem K, Fauler M, Fois G, Hellmann A, Winokurow N, Schumacher S, et al. Mechanical stretch activates piezo1 in caveolae of alveolar type I cells to trigger ATP release and paracrine stimulation of surfactant secretion from alveolar type II cells. FASEB J. 2020;34:12785–804.
Cong X, Hubmayr RD, Li C, Zhao X. Plasma membrane wounding and repair in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol. 2017;312:L371–91.
Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid Redox Signal. 2009;11:1651–67.
Ishahak M, Hill J, Amin Q, Wubker L, Hernandez A, Mitrofanova A, et al. Modular Microphysiological System for Modeling of Biologic Barrier Function. Front Bioeng Biotechnol. 2020;8:581163.
Zeinali S, Thompson EK, Gerhardt H, Geiser T, Guenat OT. Remodeling of an in vitro microvessel exposed to cyclic mechanical stretch. APL Bioeng. 2021;5:026102.
Shimizu A, Goh WH, Itai S, Hashimoto M, Miura S, Onoe H. ECM-based microchannel for culturing in vitro vascular tissues with simultaneous perfusion and stretch. Lab Chip. 2020;20:1917–27.
Anguiano M, Castilla C, Maška M, Ederra C, Peláez R, Morales X, et al. Characterization of three-dimensional cancer cell migration in mixed collagen-Matrigel scaffolds using microfluidics and image analysis. PLoS One. 2017;12:e0171417.
Nalayanda DD, Puleo C, Fulton WB, Sharpe LM, Wang T-H, Abdullah F. An open-access microfluidic model for lung-specific functional studies at an air-liquid interface. Biomed Microdevices. 2009;11:1081–9.
van Riet S, Ninaber DK, Mikkers HMM, Tetley TD, Jost CR, Mulder AA, et al. In vitro modelling of alveolar repair at the air-liquid interface using alveolar epithelial cells derived from human induced pluripotent stem cells. Sci Rep. 2020;10:5499.
Mermoud Y, Felder M, Stucki JD, Stucki AO, Guenat OT. Microimpedance tomography system to monitor cell activity and membrane movements in a breathing lung-on-chip. Sensors and Actuators B: Chemical. 2018;255:3647–53.
Bichsel CA, Hall SRR, Schmid RA, Guenat OT, Geiser T. Primary Human Lung Pericytes Support and Stabilize In Vitro Perfusable Microvessels. Tissue Eng Part A. 2015;21:2166–76.
Haak AJ, Tan Q, Tschumperlin DJ. Matrix biomechanics and dynamics in pulmonary fibrosis. Matrix Biol. 2018;73:64–76.
Sundarakrishnan A, Zukas H, Coburn J, Bertini BT, Liu Z, Georgakoudi I, et al. Bioengineered in Vitro Tissue Model of Fibroblast Activation for Modeling Pulmonary Fibrosis. ACS Biomater Sci Eng. 2019;5:2417–29.
Felder M, Trueeb B, Stucki AO, Borcard S, Stucki JD, Schnyder B, et al. Impaired Wound Healing of Alveolar Lung Epithelial Cells in a Breathing Lung-On-A-Chip. Front Bioeng Biotechnol. 2019;7:3.
Mejías JC, Nelson MR, Liseth O, Roy K. A 96-well format microvascularized human lung-on-a-chip platform for microphysiological modeling of fibrotic diseases. Lab Chip. 2020;20:3601–11.
Asmani M, Velumani S, Li Y, Wawrzyniak N, Hsia I, Chen Z, et al. Fibrotic microtissue array to predict anti-fibrosis drug efficacy. Nat Commun. 2018;9:2066.
Felder M, Trueeb B, Stucki AO, Borcard S, Stucki JD, Schnyder B, et al. Impaired Wound Healing of Alveolar Lung Epithelial Cells in a Breathing Lung-On-A-Chip. Frontiers in bioengineering and biotechnology. 2019;7:3.
Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392:2052–90.
Tuder RM, Yoshida T, Arap W, Pasqualini R, Petrache I. State of the art. Cellular and molecular mechanisms of alveolar destruction in emphysema: an evolutionary perspective. Proc Am Thorac Soc. 2006;3:503–10.
Krimmer DI, Oliver BGG. What can in vitro models of COPD tell us? Pulm Pharmacol Ther. 2011;24:471–7.
Madl AK, Plummer LE, Carosino C, Pinkerton KE. Nanoparticles, lung injury, and the role of oxidant stress. Annu Rev Physiol. 2014;76:447–65.
Zhang M, Xu C, Jiang L, Qin J. A 3D human lung-on-a-chip model for nanotoxicity testing. Toxicol Res (Camb). 2018;7:1048–60.
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33.
Nalayanda DD, Fulton WB, Colombani PM, Wang T-H, Abdullah F. Pressure induced lung injury in a novel in vitro model of the alveolar interface: protective effect of dexamethasone. J Pediatr Surg. 2014;49:61–5; discussion 65.
Xu C, Zhang M, Chen W, Jiang L, Chen C, Qin J. Assessment of Air Pollutant PM2.5 Pulmonary Exposure Using a 3D Lung-on-Chip Model. ACS Biomater Sci Eng. 2020;6:3081–90.
Deinhardt-Emmer S, Rennert K, Schicke E, Cseresnyés Z, Windolph M, Nietzsche S, et al. Co-infection with Staphylococcus aureus after primary influenza virus infection leads to damage of the endothelium in a human alveolus-on-a-chip model. Biofabrication. 2020;12:025012.
Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–40.
Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and Multiorgan Response. Curr Probl Cardiol. 2020;45:100618.
Kiener M, Roldan N, Machahua C, Sengupta A, Geiser T, Guenat OT, et al. Human-Based Advanced in vitro Approaches to Investigate Lung Fibrosis and Pulmonary Effects of COVID-19. Front Med (Lausanne). 2021;8:644678.
Deinhardt-Emmer S, Böttcher S, Häring C, Giebeler L, Henke A, Zell R, et al. SARS-CoV-2 causes severe epithelial inflammation and barrier dysfunction. J Virol. 2021;JVI.00110-21.
Zhang M, Wang P, Luo R, Wang Y, Li Z, Guo Y, et al. Biomimetic Human Disease Model of SARS-CoV-2 Induced Lung Injury and Immune Responses on Organ Chip System. Adv Sci (Weinh). 2020;2002928.
Thacker VV, Sharma K, Dhar N, Mancini G-F, Sordet-Dessimoz J, McKinney JD. Rapid endotheliitis and vascular damage characterize SARS-CoV-2 infection in a human lung-on-chip model. EMBO Rep. 2021;22:e52744.
Denisenko TV, Budkevich IN, Zhivotovsky B. Cell death-based treatment of lung adenocarcinoma. Cell Death Dis. 2018;9:117.
Singleton DC, Macann A, Wilson WR. Therapeutic targeting of the hypoxic tumour microenvironment. Nat Rev Clin Oncol. 2021;
Park S, Kim TH, Kim SH, You S, Jung Y. Three-Dimensional Vascularized Lung Cancer-on-a-Chip with Lung Extracellular Matrix Hydrogels for In Vitro Screening. Cancers (Basel). 2021;13:3930.
Piergiovanni M, Leite SB, Corvi R, Whelan M. Standardisation needs for organ on chip devices. Lab Chip. 2021;21:2857–68.
Beers MF, Moodley Y. When Is an Alveolar Type 2 Cell an Alveolar Type 2 Cell? A Conundrum for Lung Stem Cell Biology and Regenerative Medicine. Am J Respir Cell Mol Biol. 2017;57:18–27.
McMinn PH, Hind LE, Huttenlocher A, Beebe DJ. Neutrophil trafficking on-a-chip: an in vitro, organotypic model for investigating neutrophil priming, extravasation, and migration with spatiotemporal control. Lab Chip. 2019;19:3697–705.
Oleaga C, Bernabini C, Smith AST, Srinivasan B, Jackson M, McLamb W, et al. Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci Rep. 2016;6:20030.
Marsland BJ, Trompette A, Gollwitzer ES. The Gut-Lung Axis in Respiratory Disease. Ann Am Thorac Soc. 2015;12 Suppl 2:S150–156.
Mencattini A, Mattei F, Schiavoni G, Gerardino A, Businaro L, Di Natale C, et al. From Petri Dishes to Organ on Chip Platform: The Increasing Importance of Machine Learning and Image Analysis. Front Pharmacol. 2019;10:100.
Zhang B, Korolj A, Lai BFL, Radisic M. Advances in organ-on-a-chip engineering. Nat Rev Mater. 2018;3:257–78.
Kilic T, Navaee F, Stradolini F, Renaud P, Carrara S. Organs-on-chip monitoring: sensors and other strategies. Microphysiol Syst. 2018;1:1–1.
Khalid MAU, Kim YS, Ali M, Lee BG, Cho Y-J, Choi KH. A lung cancer-on-chip platform with integrated biosensors for physiological monitoring and toxicity assessment. Biochemical Engineering Journal. 2020;155:107469.
Acknowledgements
This work was supported by funding from the Swiss National Science Foundation (project: 185365), the Swiss 3R Competence Centre (project: 3RCC-OC-2019-025), the H2020 Eurostars (project: AIM4DoC) and MSCA-ITN (EUROoC, project: 812954), Innosuisse (project: 48818.1 IP-LS) and the Novartis Foundation for medical-biological Research (project: #20B146). The authors thank Dr. Anne Morbach for the illustration of Fig. 10.2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zamprogno, P. et al. (2023). Lung-on-a-Chip Models of the Lung Parenchyma. In: Magin, C.M. (eds) Engineering Translational Models of Lung Homeostasis and Disease. Advances in Experimental Medicine and Biology, vol 1413. Springer, Cham. https://doi.org/10.1007/978-3-031-26625-6_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-26625-6_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-26624-9
Online ISBN: 978-3-031-26625-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)