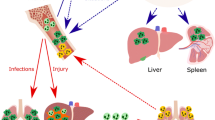
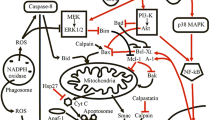
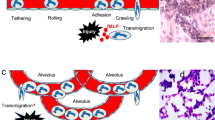
Abstract
Neutrophils are the essential guards of the immune system that inactivate different pathogens as well as instruct specific immune responses. Nitric oxide (NO), a pleiotropic signaling molecule produced from nitric oxide synthases, regulates neutrophils at diverse levels. This includes the development of neutrophils from hematopoietic stem cells through granulopoiesis processes; furthermore, nitric oxide regulates neutrophil maturation from committed progenitor cells. In addition, nitric oxide regulates most of the neutrophil functions, such as adhesion, chemotaxis, respiratory burst, apoptosis, NETosis, intruder killing, and tissue damage. Intriguingly, neutrophils provide substantial amount of NO with the presence of nitric oxide synthases and their regulation in inflammatory conditions. Overall, this work discusses the role of nitric oxide signaling in neutrophil ontogeny and functions.
Sachin Kumar and Samreen Sadaf authors have contributed equally
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Lo Celso C, Scadden DT (2011) The haematopoietic stem cell niche at a glance. J Cell Sci 124(Pt 21):3529–3535
Trumpp A, Essers M, Wilson A (2010) Awakening dormant haematopoietic stem cells. Nat Rev Immunol 10(3):201–209
Schofield R (1978) The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4(1–2):7–25
Boulais PE, Frenette PS (2015) Making sense of hematopoietic stem cell niches. Blood 125(17):2621–2629
Morrison SJ, Weissman IL (1994) The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity 1(8):661–673
Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL (1997) Identification of a lineage of multipotent hematopoietic progenitors. Development 124(10):1929–1939
Kondo M, Weissman IL, Akashi K (1997) Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91(5):661–672
Chotinantakul K, Leeanansaksiri W (2012) Hematopoietic stem cell development, niches, and signaling pathways. Bone Marrow Res 2012:270425
Borregaard N, Cowland JB (1997) Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89(10):3503–3521
Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE (1997) Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci USA 94(10):5320–5325
Conneally E, Cashman J, Petzer A, Eaves C (1997) Expansion in vitro of transplantable human cord blood stem cells demonstrated using a quantitative assay of their lympho-myeloid repopulating activity in nonobese diabetic-scid/scid mice. Proc Natl Acad Sci USA 94(18):9836–9841
Gammaitoni L, Bruno S, Sanavio F, Gunetti M, Kollet O, Cavalloni G et al (2003) Ex vivo expansion of human adult stem cells capable of primary and secondary hemopoietic reconstitution. Exp Hematol 31(3):261–270
Miller CL, Eaves CJ (1997) Expansion in vitro of adult murine hematopoietic stem cells with transplantable lympho-myeloid reconstituting ability. Proc Natl Acad Sci USA 94(25):13648–13653
Zandstra PW, Conneally E, Petzer AL, Piret JM, Eaves CJ (1997) Cytokine manipulation of primitive human hematopoietic cell self-renewal. Proc Natl Acad Sci USA 94(9):4698–4703
Metcalf D (2008) Hematopoietic cytokines. Blood 111(2):485–491
Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C et al (1994) Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood 84(6):1737–1746
Liu F, Wu HY, Wesselschmidt R, Kornaga T, Link DC (1996) Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient mice. Immunity 5(5):491–501
Christopherson KW 2nd, Cooper S, Broxmeyer HE (2003) Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood 101(12):4680–4686
Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ (2001) Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood 98(5):1289–1297
Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR et al (2002) Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 109(5):625–637
Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L et al (2002) G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol 3(7):687–694
Winkler IG, Hendy J, Coughlin P, Horvath A, Levesque JP (2005) Serine protease inhibitors serpina1 and serpina3 are down-regulated in bone marrow during hematopoietic progenitor mobilization. J Exp Med 201(7):1077–1088
Nagase H, Miyamasu M, Yamaguchi M, Imanishi M, Tsuno NH, Matsushima K et al (2002) Cytokine-mediated regulation of CXCR4 expression in human neutrophils. J Leukoc Biol 71(4):711–717
Kim HK, De La Luz SM, Williams CK, Gulino AV, Tosato G (2006) G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood 108(3):812–820
Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA et al (2004) Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood 104(2):565–571
Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM (2003) Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19(4):583–593
Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F et al (2003) Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet 34(1):70–74
Eash KJ, Means JM, White DW, Link DC (2009) CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 113(19):4711–4719
von Vietinghoff S, Ley K (2008) Homeostatic regulation of blood neutrophil counts. J Immunol 181(8):5183–5188
Ma Q, Jones D, Springer TA (1999) The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity 10(4):463–471
Moncada S, Palmer RM, Higgs EA (1989) Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol 38(11):1709–15
Gross SS, Jaffe EA, Levi R, Kilbourn RG (1991) Cytokine-activated endothelial cells express an isotype of nitric oxide synthase which is tetrahydrobiopterin-dependent, calmodulin-independent and inhibited by arginine analogs with a rank-order of potency characteristic of activated macrophages. Biochem Biophys Res Commun 178(3):823–829
Bredt DS, Snyder SH (1994) Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 63:175–195
Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357(Pt 3):593–615
Helfrich MH, Evans DE, Grabowski PS, Pollock JS, Ohshima H, Ralston SH (1997) Expression of nitric oxide synthase isoforms in bone and bone cell cultures. J Bone Miner Res 12(7):1108–1115
Morton J, Coles B, Wright K, Gallimore A, Morrow JD, Terry ES et al (2008) Circulating neutrophils maintain physiological blood pressure by suppressing bacteria and IFNgamma-dependent iNOS expression in the vasculature of healthy mice. Blood 111(10):5187–5194
Srivastava P, Rajanikanth M, Raghavan SA, Dikshit M (2002) Role of endogenous reactive oxygen derived species and cyclooxygenase mediators in 5-hydroxytryptamine-induced contractions in rat aorta: relationship to nitric oxide. Pharmacol Res 45(5):375–382
Saini R, Patel S, Saluja R, Sahasrabuddhe AA, Singh MP, Habib S et al (2006) Nitric oxide synthase localization in the rat neutrophils: immunocytochemical, molecular, and biochemical studies. J Leukoc Biol 79(3):519–528
Saluja R, Bajpai VK, Mitra K, Habib S, Dikshit M (2007) Molecular characterization and distribution of nitric oxide synthase in human blood cells. Paper presented at Emerging trends in free radical and antioxidant research, Third Meeting of the Society for Free Radical Research-Asia (SFRR-Asia) & Sixth annual meeting of Society for Free Radical Research-India (SFRR-India), Mumbai, Lonavala, India, 177p
Chatterjee M, Saluja R, Tewari S, Barthwal MK, Goel SK, Dikshit M (2009) Augmented nitric oxide generation in neutrophils: oxidative and pro-inflammatory implications in hypertension. Free Radic Res 43(12):1195–1204
Saluja R, Saini R, Mitra K, Bajpai VK, Dikshit M (2010) Ultrastructural immunogold localization of nitric oxide synthase isoforms in rat and human eosinophils. Cell Tissue Res 340(2):381–388
Jyoti A, Singh AK, Dubey M, Kumar S, Saluja R, Keshari RS et al (2014) Interaction of inducible nitric oxide synthase with rac2 regulates reactive oxygen and nitrogen species generation in the human neutrophil phagosomes: implication in microbial killing. Antioxid Redox Signal 20(3):417–431
Jalnapurkar S, Singh S, Devi MR, Limaye L, Kale V (2016) Nitric oxide has contrasting age-dependent effects on the functionality of murine hematopoietic stem cells. Stem Cell Res Ther 7(1):171
North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM et al (2009) Hematopoietic stem cell development is dependent on blood flow. Cell 137(4):736–748
Sadaf S, Awasthi D, Singh AK, Nagarkoti S, Kumar S, Barthwal MK, et al (2019) Pyroptotic and apoptotic cell death in iNOS and nNOS overexpressing K562 cells: a mechanistic insight. Biochem Pharmacol:113779.
Sadaf S, Singh AK, Awasthi D, Nagarkoti S, Agrahari AK, Srivastava RN, et al (2019) Augmentation of iNOS expression in myeloid progenitor cells expedites neutrophil differentiation. J Leukoc Biol
Shami PJ, Weinberg JB (1996) Differential effects of nitric oxide on erythroid and myeloid colony growth from CD34+ human bone marrow cells. Blood 87(3):977–982
Ishida A, Sasaguri T, Kosaka C, Nojima H, Ogata J (1997) Induction of the cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1) by nitric oxide-generating vasodilator in vascular smooth muscle cells. J Biol Chem 272(15):10050–10057
Kumar S, Jyoti A, Keshari RS, Singh M, Barthwal MK, Dikshit M (2010) Functional and molecular characterization of NOS isoforms in rat neutrophil precursor cells. Cytometry A 77(5):467–477
Enikolopov G, Banerji J, Kuzin B (1999) Nitric oxide and Drosophila development. Cell Death Differ 6(10):956–963
Kumar S, Barthwal MK, Dikshit M (2010) Cdk2 nitrosylation and loss of mitochondrial potential mediate NO-dependent biphasic effect on HL-60 cell cycle. Free Radical Biol Med 48(6):851–861
Punjabi CJ, Laskin DL, Heck DE, Laskin JD (1992) Production of nitric oxide by murine bone marrow cells. Inverse correlation with cellular proliferation. J Immunol 149(6):2179–84
Maciejewski JP, Selleri C, Sato T, Cho HJ, Keefer LK, Nathan CF, et al (1995) Nitric oxide suppression of human hematopoiesis in vitro: contribution to inhibitory action of interferon-gamma and tumor necrosis factor-alpha. J Clin Invest 96(2):1085–92
Nolan S, Dixon R, Norman K, Hellewell P, Ridger V (2008) Nitric oxide regulates neutrophil migration through microparticle formation. Am J Pathol 172(1):265–273
Krasnov P, Michurina T, Packer MA, Stasiv Y, Nakaya N, Moore KA et al (2008) Neuronal nitric oxide synthase contributes to the regulation of hematopoiesis. Mol Med 14(3–4):141–149
Noiri E, Lee E, Testa J, Quigley J, Colflesh D, Keese CR et al (1998) Podokinesis in endothelial cell migration: role of nitric oxide. Am J Physiol 274(1):C236–C244
Punjabi CJ, Laskin JD, Hwang SM, MacEachern L, Laskin DL (1994) Enhanced production of nitric oxide by bone marrow cells and increased sensitivity to macrophage colony-stimulating factor (CSF) and granulocyte-macrophage CSF after benzene treatment of mice. Blood 83(11):3255–3263
Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K et al (2003) Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 9(11):1370–1376
Nogueira-Pedro A, Dias CC, Regina H, Segreto C, Addios PC, Lungato L et al (2014) Nitric oxide-induced murine hematopoietic stem cell fate involves multiple signaling proteins, gene expression, and redox modulation. Stem Cells 32(11):2949–2960
Suda T, Arai F, Hirao A (2005) Hematopoietic stem cells and their niche. Trends Immunol 26(8):426–433
Sethi S, Dikshit M (2000) Modulation of polymorphonuclear leukocytes function by nitric oxide. Thromb Res 100(3):223–247
Michurina T, Krasnov P, Balazs A, Nakaya N, Vasilieva T, Kuzin B et al (2004) Nitric oxide is a regulator of hematopoietic stem cell activity. Mol Ther 10(2):241–248
Dubey M, Nagarkoti S, Awasthi D, Singh AK, Chandra T, Kumaravelu J et al (2016) Nitric oxide-mediated apoptosis of neutrophils through caspase-8 and caspase-3-dependent mechanism. Cell Death Dis 7(9):e2348
Chenais B, Molle I, Jeannesson P (1999) Inhibitory effect of nitric oxide on chemically induced differentiation of human leukemic K562 cells. Biochem Pharmacol 58(5):773–778
Shami PJ, Sauls DL, Weinberg JB (1998) Schedule and concentration-dependent induction of apoptosis in leukemia cells by nitric oxide. Leukemia 12(9):1461–1466
Magrinat G, Mason SN, Shami PJ, Weinberg JB (1992) Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood 80(8):1880–1884
Diaz MF, Li N, Lee HJ, Adamo L, Evans SM, Willey HE et al (2015) Biomechanical forces promote blood development through prostaglandin E2 and the cAMP-PKA signaling axis. J Exp Med 212(5):665–680
Nasrallah R, Knezevic K, Thai T, Thomas SR, Gottgens B, Lacaud G et al (2015) Endoglin potentiates nitric oxide synthesis to enhance definitive hematopoiesis. Biol Open 4(7):819–829
Tejedo JR, Tapia-Limonchi R, Mora-Castilla S, Cahuana GM, Hmadcha A, Martin F et al (2010) Low concentrations of nitric oxide delay the differentiation of embryonic stem cells and promote their survival. Cell Death Dis 1:e80
Mora-Castilla S, Tejedo JR, Hmadcha A, Cahuana GM, Martin F, Soria B et al (2010) Nitric oxide repression of Nanog promotes mouse embryonic stem cell differentiation. Cell Death Differ 17(6):1025–1033
Trento C, Marigo I, Pievani A, Galleu A, Dolcetti L, Wang CY et al (2017) Bone marrow mesenchymal stromal cells induce nitric oxide synthase-dependent differentiation of CD11b(+) cells that expedite hematopoietic recovery. Haematologica 102(5):818–825
Tiribuzi R, Crispoltoni L, Tartacca F, Orlacchio A, Martino S, Palmerini CA et al (2013) Nitric oxide depletion alters hematopoietic stem cell commitment toward immunogenic dendritic cells. Biochim Biophys Acta 1830(3):2830–2838
Iversen PO, Nicolaysen G, Benestad HB (1994) Endogenous nitric oxide causes vasodilation in rat bone marrow, bone, and spleen during accelerated hematopoiesis. Exp Hematol 22(13):1297–1302
Sadaf S, Singh AK, Awasthi D, Nagarkoti S, Agrahari AK, Srivastava RN et al (2019) Augmentation of iNOS expression in myeloid progenitor cells expedites neutrophil differentiation. J Leukoc Biol 106(2):397–412
Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K et al (2004) Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118(2):149–161
Cui X, Chen J, Zacharek A, Roberts C, Yang Y, Chopp M (2009) Nitric oxide donor up-regulation of SDF1/CXCR4 and Ang1/Tie2 promotes neuroblast cell migration after stroke. J Neurosci Res 87(1):86–95
Zhang Y, Wittner M, Bouamar H, Jarrier P, Vainchenker W, Louache F (2007) Identification of CXCR4 as a new nitric oxide-regulated gene in human CD34+ cells. Stem Cells 25(1):211–219
Lapidot T, Petit I (2002) Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol 30(9):973–981
Hall CJ, Flores MV, Oehlers SH, Sanderson LE, Lam EY, Crosier KE et al (2012) Infection-responsive expansion of the hematopoietic stem and progenitor cell compartment in zebrafish is dependent upon inducible nitric oxide. Cell Stem Cell 10(2):198–209
Rossner S, Voigtlander C, Wiethe C, Hanig J, Seifarth C, Lutz MB (2005) Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur J Immunol 35(12):3533–3544
Aicher A, Heeschen C, Dimmeler S (2004) The role of NOS3 in stem cell mobilization. Trends Mol Med 10(9):421–425
Jovcic G, Bugarski D, Petakov M, Krstic A, Vlaski M, Stojanovic N et al (2004) In vivo effects of interleukin-17 on haematopoietic cells and cytokine release in normal mice. Cell Prolif 37(6):401–412
Wang CH, Hsieh WY, Shih LY, Lin HC, Liu CY, Chung KF et al (1999) Increased progenitor cell proliferation in the peripheral blood of patients with bronchial asthma: the role of nitric oxide. J Allergy Clin Immunol 104(4 Pt 1):803–810
Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P et al (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature 362(6418):318–324
Kumar S, Patel S, Jyoti A, Keshari RS, Verma A, Barthwal MK et al (2010) Nitric oxide-mediated augmentation of neutrophil reactive oxygen and nitrogen species formation: critical use of probes. Cytometry A 77(11):1038–1048
Kumar S, Barthwal MK, Dikshit M (2011) Nitrite-mediated modulation of HL-60 cell cycle and proliferation: involvement of cyclin-dependent kinase 2 activation. J Pharmacol Exp Ther 337(3):812–821
Salvemini D, de Nucci G, Gryglewski RJ, Vane JR (1989) Human neutrophils and mononuclear cells inhibit platelet aggregation by releasing a nitric oxide-like factor. Proc Natl Acad Sci USA 86(16):6328–6332
Wright CD, Mulsch A, Busse R, Osswald H (1989) Generation of nitric oxide by human neutrophils. Biochem Biophys Res Commun 160(2):813–819
Rimele TJ, Sturm RJ, Adams LM, Henry DE, Heaslip RJ, Weichman BM et al (1988) Interaction of neutrophils with vascular smooth muscle: identification of a neutrophil-derived relaxing factor. J Pharmacol Exp Ther 245(1):102–111
Dikshit M, Kumari R, Srimal RC (1993) Pulmonary thromboembolism-induced alterations in nitric oxide release from rat circulating neutrophils. J Pharmacol Exp Ther 265(3):1369–1373
Chen LY, Mehta JL (1996) Further evidence of the presence of constitutive and inducible nitric oxide synthase isoforms in human platelets. J Cardiovasc Pharmacol 27(1):154–158
Faint RW, Mackie IJ, Machin SJ (1991) Platelet aggregation is inhibited by a nitric oxide-like factor released from human neutrophils in vitro. Br J Haematol 77(4):539–545
Sethi S, Singh MP, Dikshit M (1999) Nitric oxide-mediated augmentation of polymorphonuclear free radical generation after hypoxia-reoxygenation. Blood 93(1):333–340
Miles AM, Owens MW, Milligan S, Johnson GG, Fields JZ, Ing TS, et al (1995) Nitric oxide synthase in circulating vs. extravasated polymorphonuclear leukocytes. J Leukoc Biol 58(5):616–22
Evans TJ, Buttery LD, Carpenter A, Springall DR, Polak JM, Cohen J (1996) Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc Natl Acad Sci U S A 93(18):9553–9558
Wheeler MA, Smith SD, Garcia-Cardena G, Nathan CF, Weiss RM, Sessa WC (1997) Bacterial infection induces nitric oxide synthase in human neutrophils. J Clin Invest 99(1):110–116
Cedergren J, Follin P, Forslund T, Lindmark M, Sundqvist T, Skogh T (2003) Inducible nitric oxide synthase (NOS II) is constitutive in human neutrophils. APMIS 111(10):963–968
Greenberg SS, Ouyang J, Zhao X, Giles TD (1998) Human and rat neutrophils constitutively express neural nitric oxide synthase mRNA. Nitric Oxide 2(3):203–212
Greenberg SS, **e J, Zhao X, Jie O, Giles TD (1996) An in vivo cytokine and endotoxin-independent pathway for induction of nitric oxide synthase II mRNA, enzyme, and nitrate/nitrite in alveolar macrophages. Biochem Biophys Res Commun 227(1):160–167
Wallerath T, Gath I, Aulitzky WE, Pollock JS, Kleinert H, Forstermann U (1997) Identification of the NO synthase isoforms expressed in human neutrophil granulocytes, megakaryocytes and platelets. Thromb Haemost 77(1):163–167
de F, Sanchez de Miguel L, Farre J, Gomez J, Romero J, Marcos-Alberca P, et al (2001) Expression of an endothelial-type nitric oxide synthase isoform in human neutrophils: modification by tumor necrosis factor-alpha and during acute myocardial infarction. J Am Coll Cardiol 37(3):800–7
Gatto EM, Riobo NA, Carreras MC, Chernavsky A, Rubio A, Satz ML et al (2000) Overexpression of neutrophil neuronal nitric oxide synthase in Parkinson’s disease. Nitric Oxide 4(5):534–539
Chatterjee M, Saluja R, Kumar V, Jyoti A, Kumar Jain G, Kumar Barthwal M, et al (2008) Ascorbate sustains neutrophil NOS expression, catalysis, and oxidative burst. Free Radic Biol Med
Chatterjee M, Saluja R, Kanneganti S, Chinta S, Dikshit M (2007) Biochemical and molecular evaluation of neutrophil NOS in spontaneously hypertensive rats. Cell Mol Biol (Noisy-le-grand) 53(1):84–93
Sethi S, Sharma P, Dikshit M (2001) Nitric oxide- and oxygen-derived free radical generation from control and lipopolysaccharide-treated rat polymorphonuclear leukocyte. Nitric Oxide 5(5):482–493
Seth P, Kumari R, Dikshit M, Srimal RC (1994) Modulation of rat peripheral polymorphonuclear leukocyte response by nitric oxide and arginine. Blood 84(8):2741–2748
Seth P, Kumari R, Dikshit M (1997) Alterations in the free radical generation and nitric oxide release from rat peripheral polymorphonuclear leukocytes following thrombosis. Thromb Res 87(3):279–288
Barthwal MK, Srivastava N, Shukla R, Nag D, Seth PK, Srimal RC et al (1999) Polymorphonuclear leukocyte nitrite content and antioxidant enzymes in Parkinson’s disease patients. Acta Neurol Scand 100(5):300–304
Dikshit M, Sharma P (2002) Nitric oxide mediated modulation of free radical generation response in the rat polymorphonuclear leukocytes: a flowcytometric study. Methods Cell Sci 24(1–3):69–76
Sharma P, Raghavan SA, Dikshit M (2003) Role of ascorbate in the regulation of nitric oxide generation by polymorphonuclear leukocytes. Biochem Biophys Res Commun 309(1):12–17
Chatterjee M, Saluja R, Kumar V, Jyoti A, Kumar Jain G, Kumar M et al (2008) Ascorbate sustains neutrophil NOS expression, catalysis, and oxidative burst. Free Radical Biol Med 45(8):1084–1093
Sadaf S, Awasthi D, Singh AK, Nagarkoti S, Kumar S, Barthwal MK et al (2020) Pyroptotic and apoptotic cell death in iNOS and nNOS overexpressing K562 cells: a mechanistic insight. Biochem Pharmacol 176:113779
Neilly IJ, Copland M, Haj M, Adey G, Benjamin N, Bennett B (1995) Plasma nitrate concentrations in neutropenic and non-neutropenic patients with suspected septicaemia. Br J Haematol 89(1):199–202
Kothari N, Bogra J, Kohli M, Malik A, Kothari D, Srivastava S et al (2012) Role of active nitrogen molecules in progression of septic shock. Acta Anaesthesiol Scand 56(3):307–315
Kothari N, Keshari RS, Bogra J, Kohli M, Abbas H, Malik A, et al (2011) Increased myeloperoxidase enzyme activity in plasma is an indicator of inflammation and onset of sepsis. J Crit Care 26(4):435 e1–7
Babu CK, Ansari KM, Mehrotra S, Patel S, Dikshit M, Das M (2010) Activation of inflammatory response and apoptosis of polymorphonuclear leukocytes in patients with argemone oil poisoning. Chem Biol Interact 183(1):154–164
Kumar S, Dikshit M (2019) Reactive oxygen species generation in neutrophils: modulation by nitric oxide. Springer, Singapore
Jain M, Kumar A, Singh US, Kushwaha R, Singh AK, Dikshit M et al (2017) Cellular and plasma nitrite levels in myeloid leukemia: a pathogenetic decrease. Biol Chem 398(11):1259–1265
Singh AK, Awasthi D, Dubey M, Nagarkoti S, Kumar A, Chandra T et al (2016) High oxidative stress adversely affects NFkappaB mediated induction of inducible nitric oxide synthase in human neutrophils: implications in chronic myeloid leukemia. Nitric Oxide 58:28–41
Shukla R, Rajani M, Srivastava N, Barthwal MK, Dikshit M (2006) Nitrite and malondialdehyde content in cerebrospinal fluid of patients with Parkinson’s disease. Int J Neurosci 116(12):1391–1402
Srivastava N, Barthwal MK, Dalal PK, Agarwal AK, Nag D, Srimal RC et al (2001) Nitrite content and antioxidant enzyme levels in the blood of schizophrenia patients. Psychopharmacology 158(2):140–145
Srivastava N, Barthwal MK, Dalal PK, Agarwal AK, Nag D, Seth PK et al (2002) A study on nitric oxide, beta-adrenergic receptors and antioxidant status in the polymorphonuclear leukocytes from the patients of depression. J Affect Disord 72(1):45–52
Shukla R, Barthwal MK, Srivastava N, Nag D, Seth PK, Srimal RC et al (2001) Blood nitrite levels in patients with migraine during headache-free period. Headache 41(5):475–481
Shukla R, Rajani M, Barthwal MK, Srivastava N, Dikshit M (2003) Cerebrospinal fluid nitrite and malondialdehyde levels in patients with motor neuron disease. Int J Neurosci 113(8):1043–1054
Shukla R, Barthwal MK, Srivastava N, Sharma P, Raghavan SA, Nag D et al (2004) Neutrophil-free radical generation and enzymatic antioxidants in migraine patients. Cephalalgia 24(1):37–43
Kumar S, Dikshit M (2019) Metabolic insight of neutrophils in health and disease. Front Immunol 10:2099
Pontremoli S, Salamino F, Sparatore B, De Tullio R, Patrone M, Tizianello A et al (1989) Enhanced activation of the respiratory burst oxidase in neutrophils from hypertensive patients. Biochem Biophys Res Commun 158(3):966–972
Malawista SE, Montgomery RR, van Blaricom G (1992) Evidence for reactive nitrogen intermediates in killing of staphylococci by human neutrophil cytoplasts: a new microbicidal pathway for polymorphonuclear leukocytes. J Clin Invest 90(2):631–6
Davis KL, Martin E, Turko IV, Murad F (2001) Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol 41:203–236
Li CQ, Wogan GN (2005) Nitric oxide as a modulator of apoptosis. Cancer Lett 226(1):1–15
Taylor EL, Megson IL, Haslett C, Rossi AG (2003) Nitric oxide: a key regulator of myeloid inflammatory cell apoptosis. Cell Death Differ 10(4):418–430
Akgul C, Moulding DA, Edwards SW (2001) Molecular control of neutrophil apoptosis. FEBS Lett 487(3):318–322
Dubey M, Singh AK, Awasthi D, Nagarkoti S, Kumar S, Ali W et al (2015) L-Plastin S-glutathionylation promotes reduced binding to beta-actin and affects neutrophil functions. Free Radical Biol Med 86:1–15
Awasthi D, Nagarkoti S, Sadaf S, Chandra T, Kumar S, Dikshit M (2019) Glycolysis dependent lactate formation in neutrophils: a metabolic link between NOX-dependent and independent NETosis. Biochim Biophys Acta Mol Basis Dis 1865(12):165542
Kubes P, Suzuki M, Granger DN (1991) Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA 88(11):4651–4655
Akimitsu T, Gute DC, Korthuis RJ (1995) Leukocyte adhesion induced by inhibition of nitric oxide production in skeletal muscle. J Appl Physiol 78(5):1725–1732
Mitchell DJ, Yu J, Tyml K (1998) Local L-NAME decreases blood flow and increases leukocyte adhesion via CD18. Am J Physiol 274(4 Pt 2):H1264–H1268
Granger DN, Kubes P (1994) The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol 55(5):662–675
Terada LS (1996) Hypoxia-reoxygenation increases O2: efflux which injures endothelial cells by an extracellular mechanism. Am J Physiol 270(3 Pt 2):H945–50
Johnston B, Kanwar S, Kubes P (1996) Hydrogen peroxide induces leukocyte rolling: modulation by endogenous antioxidant mechanisms including NO. Am J Physiol 271(2 Pt 2):H614–H621
Gaboury J, Woodman RC, Granger DN, Reinhardt P, Kubes P (1993) Nitric oxide prevents leukocyte adherence: role of superoxide. Am J Physiol 265(3 Pt 2):H862–H867
Hickey MJ, Sharkey KA, Sihota EG, Reinhardt PH, Macmicking JD, Nathan C et al (1997) Inducible nitric oxide synthase-deficient mice have enhanced leukocyte-endothelium interactions in endotoxemia. FASEB J Official Publ Fed Am Soc Exp Biol 11(12):955–964
Dal Secco D, Moreira AP, Freitas A, Silva JS, Rossi MA, Ferreira SH et al (2006) Nitric oxide inhibits neutrophil migration by a mechanism dependent on ICAM-1: role of soluble guanylate cyclase. Nitric Oxide 15(1):77–86
Oka S, Sasada M, Yamamoto K, Nohgawa M, Takahashi A, Yamashita K et al (2005) Nitric oxide derived from human umbilical vein endothelial cells inhibits transendothelial migration of neutrophils. Int J Hematol 81(3):220–227
Forslund T, Nilsson HM, Sundqvist T (2000) Nitric oxide regulates the aggregation of stimulated human neutrophils. Biochem Biophys Res Commun 274(2):482–487
Banick PD, Chen Q, Xu YA, Thom SR (1997) Nitric oxide inhibits neutrophil beta 2 integrin function by inhibiting membrane-associated cyclic GMP synthesis. J Cell Physiol 172(1):12–24
Kosonen O, Kankaanranta H, Malo-Ranta U, Moilanen E (1999) Nitric oxide-releasing compounds inhibit neutrophil adhesion to endothelial cells. Eur J Pharmacol 382(2):111–117
Gluckman TL, Grossman JE, Folts JD, Kruse-Elliott KT (2000) Regulation of leukocyte function by nitric oxide donors: the effect of S-nitroso-thiol complexes. J Toxicol Environ Health A 61(1):9–26
Pernow J, Uriuda Y, Wang QD, Li XS, Nordlander R, Rydeen L (1994) The protective effect of L-arginine on myocardial injury and endothelial function following ischaemia and reperfusion in the pig. Eur Heart J 15(12):1712–1719
Nakanishi K, Vinten-Johansen J, Lefer DJ, Zhao Z, Fowler WC 3rd, McGee DS et al (1992) Intracoronary L-arginine during reperfusion improves endothelial function and reduces infarct size. Am J Physiol 263(6 Pt 2):H1650–H1658
Siegfried MR, Erhardt J, Rider T, Ma XL, Lefer AM (1992) Cardioprotection and attenuation of endothelial dysfunction by organic nitric oxide donors in myocardial ischemia-reperfusion. J Pharmacol Exp Ther 260(2):668–675
Kurose I, Wolf R, Grisham MB, Aw TY, Specian RD, Granger DN (1995) Microvascular responses to inhibition of nitric oxide production role of active oxidants. Circ Res 76(1):30–39
Sato H, Zhao ZQ, Vinten-Johansen J (1996) L-Arginine inhibits neutrophil adherence and coronary artery dysfunction. Cardiovasc Res 31(1):63–72
Fukatsu K, Saito H, Han I, Furukawa S, Lin MT, Matsuda T et al (1998) Nitric oxide donor decreases neutrophil adhesion in both lung and peritoneum during peritonitis. J Surg Res 74(2):119–124
Okayama N, Ichikawa H, Coe L, Itoh M, Alexander JS (1998) Exogenous NO enhances hydrogen peroxide-mediated neutrophil adherence to cultured endothelial cells. Am J Physiol 274(5 Pt 1):L820–L826
Okayama N, Coe L, Itoh M, Alexander JS (1999) Exogenous nitric oxide increases neutrophil adhesion to cultured human endothelial monolayers through a protein kinase G dependent mechanism. Inflammation 23(1):37–50
VanUffelen BE, de Koster BM, Van den Broek PJ, VanSteveninck J, Elferink JG (1996) Modulation of neutrophil migration by exogenous gaseous nitric oxide. J Leukoc Biol 60(1):94–100
Crosara-Alberto DP, Darini AL, Inoue RY, Silva JS, Ferreira SH, Cunha FQ (2002) Involvement of NO in the failure of neutrophil migration in sepsis induced by Staphylococcus aureus. Br J Pharmacol 136(5):645–658
Benjamim CF, Silva JS, Fortes ZB, Oliveira MA, Ferreira SH, Cunha FQ (2002) Inhibition of leukocyte rolling by nitric oxide during sepsis leads to reduced migration of active microbicidal neutrophils. Infect Immun 70(7):3602–3610
Ajuebor MN, Virag L, Flower RJ, Perretti M, Szabo C (1998) Role of inducible nitric oxide synthase in the regulation of neutrophil migration in zymosan-induced inflammation. Immunology 95(4):625–630
Wanikiat P, Woodward DF, Armstrong RA (1997) Investigation of the role of nitric oxide and cyclic GMP in both the activation and inhibition of human neutrophils. Br J Pharmacol 122(6):1135–1145
Kaplan SS, Billiar T, Curran RD, Zdziarski UE, Simmons RL, Basford RE (1989) Inhibition of chemotaxis NG-monomethyl-L-arginine: a role for cyclic GMP. Blood 74(6):1885–1887
Beauvais F, Michel L, Dubertret L (1995) Exogenous nitric oxide elicits chemotaxis of neutrophils in vitro. J Cell Physiol 165(3):610–614
Malawista SE, de Boisfleury CA (1997) Chemotaxis by human neutrophils and their cytokineplasts treated with inhibitors of nitric oxide synthase: no suppression of orientation or trajectory. J Leukoc Biol 61(1):58–62
Corriveau CC, Madara PJ, Van Dervort AL, Tropea MM, Wesley RA, Danner RL (1998) Effects of nitric oxide on chemotaxis and endotoxin-induced interleukin-8 production in human neutrophils. J Infect Dis 177(1):116–126
Moffat FL Jr, Han T, Li ZM, Peck MD, Jy W, Ahn YS et al (1996) Supplemental L-arginine HCl augments bacterial phagocytosis in human polymorphonuclear leukocytes. J Cell Physiol 168(1):26–33
Nagarkoti S, Sadaf S, Awasthi D, Chandra T, Jagavelu K, Kumar S et al (2019) L-Arginine and tetrahydrobiopterin supported nitric oxide production is crucial for the microbicidal activity of neutrophils. Free Radic Res 53(3):281–292
Forslund T, Sundqvist T (1997) Nitric oxide-releasing particles inhibit phagocytosis in human neutrophils. Biochem Biophys Res Commun 233(2):492–495
Saha P, Yeoh BS, Olvera RA, **ao X, Singh V, Awasthi D et al (2017) Bacterial siderophores hijack neutrophil functions. J Immunol 198(11):4293–4303
Sengelov H, Kjeldsen L, Borregaard N (1993) Control of exocytosis in early neutrophil activation. J Immunol 150(4):1535–1543
Moilanen E, Vuorinen P, Kankaanranta H, Metsa-Ketela T, Vapaatalo H (1993) Inhibition by nitric oxide-donors of human polymorphonuclear leucocyte functions. Br J Pharmacol 109(3):852–858
VanUffelen BE, VanSteveninck J, Elferink JG (1997) Potentiation and inhibition of fMLP-activated exocytosis in neutrophils by exogenous nitric oxide. Immunopharmacology 37(2–3):257–267
Ignarro LJ (1974) Stimulation of phagocytic release of neutral protease from human neutrophils by cholinergic amines and cyclic 3’,5’-guanosine monophosphate. J Immunol 112(1):210–214
Bladridge CW, Gerard RW (1932) The extra respiration of phagocytes. Am J Physiol 103(3):235–236
Babior BM, Kipnes RS, Curnutte JT (1973) Biological defense mechanisms: the production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest 52(3):741–4
Babior BM, Lambeth JD, Nauseef W (2002) The neutrophil NADPH oxidase. Arch Biochem Biophys 397(2):342–344
Segal AW (2005) How neutrophils kill microbes. Annu Rev Immunol 23:197–223
Sharma P, Barthwal MK, Dikshit M (2002) NO synthesis and its regulation in the arachidonic-acid-stimulated rat polymorphonuclear leukocytes. Nitric Oxide 7(2):119–126
Nagarkoti S, Dubey M, Awasthi D, Kumar V, Chandra T, Kumar S et al (2018) S-Glutathionylation of p47phox sustains superoxide generation in activated neutrophils. Biochim Biophys Acta 1865(2):444–454
Raghavan SA, Sharma P, Dikshit M (2003) Role of ascorbic acid in the modulation of inhibition of platelet aggregation by polymorphonuclear leukocytes. Thromb Res 110(2–3):117–126
Patel S, Vemula J, Konikkat S, Barthwal MK, Dikshit M (2009) Ion channel modulators mediated alteration in NO-induced free radical generation and neutrophil membrane potential. Free Radical Res
Clancy RM, Leszczynska-Piziak J, Abramson SB (1992) Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest 90(3):1116–1121
Fujii H, Ichimori K, Hoshiai K, Nakazawa H (1997) Nitric oxide inactivates NADPH oxidase in pig neutrophils by inhibiting its assembling process. J Biol Chem 272(52):32773–32778
Lee C, Miura K, Liu X, Zweier JL (2000) Biphasic regulation of leukocyte superoxide generation by nitric oxide and peroxynitrite. J Biol Chem 275(50):38965–38972
Klink M, Jastrzembska K, Bednarska K, Banasik M, Sulowska Z (2009) Effect of nitric oxide donors on NADPH oxidase signaling pathway in human neutrophils in vitro. Immunobiology
Roos D, Winterbourn CC (2002) Lethal weapons. Science 296(5568):669–671
Kim YM, Kim TH, Seol DW, Talanian RV, Billiar TR (1998) Nitric oxide suppression of apoptosis occurs in association with an inhibition of Bcl-2 cleavage and cytochrome c release. J Biol Chem 273(47):31437–31441
Luo HR, Loison F (2008) Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol 83(4):288–295
Kim KM, Kim PK, Kwon YG, Bai SK, Nam WD, Kim YM (2002) Regulation of apoptosis by nitrosative stress. J Biochem Mol Biol 35(1):127–133
Misso NL, Peacock CD, Watkins DN, Thompson PJ (2000) Nitrite generation and antioxidant effects during neutrophil apoptosis. Free Radical Biol Med 28(6):934–943
Ward C, Wong TH, Murray J, Rahman I, Haslett C, Chilvers ER et al (2000) Induction of human neutrophil apoptosis by nitric oxide donors: evidence for a caspase-dependent, cyclic-GMP-independent, mechanism. Biochem Pharmacol 59(3):305–314. Article no S0006295299003299
Blaylock MG, Cuthbertson BH, Galley HF, Ferguson NR, Webster NR (1998) The effect of nitric oxide and peroxynitrite on apoptosis in human polymorphonuclear leukocytes. Free Radic Biol Med 25(6):748–752. Article no S0891584998001087
Fortenberry JD, Owens ML, Brown LAS (1999) Am J Physiol-Lung Cell Mol Physiol 276(3):L435–L442. https://doi.org/10.1152/ajplung.1999.276.3.L435
Shibata T, Nagata K, Kobayashi Y (2007) Cutting edge: a critical role of nitric [corrected] oxide in preventing inflammation upon apoptotic cell clearance. J Immunol 179(6):3407–3411
Nagarkoti S, Dubey M, Sadaf S, Awasthi D, Chandra T, Jagavelu K et al (2019) Catalase S-Glutathionylation by NOX2 and mitochondrial-derived ROS adversely affects mice and human neutrophil survival. Inflammation 42(6):2286–2296
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS et al (2004) Neutrophil extracellular traps kill bacteria. Science 303(5663):1532–1535
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V et al (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176(2):231–241
Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD et al (2014) Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 15(11):1017–1025
Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, et al (2011) Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood 117(3):953–9
Nakashima K, Hagiwara T, Yamada M (2002) Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J Biol Chem 277(51):49562–49568
Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L et al (2004) Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 306(5694):279–283
Li JM, Fan LM, George VT, Brooks G (2007) Nox2 regulates endothelial cell cycle arrest and apoptosis via p21cip1 and p53. Free Radic Biol Med 43(6):976–986
Keshari RS, Jyoti A, Dubey M, Kothari N, Kohli M, Bogra J et al (2012) Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS ONE 7(10):e48111
Rossaint J, Herter JM, Van Aken H, Napirei M, Doring Y, Weber C et al (2014) Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood 123(16):2573–2584
Patel S, Kumar S, Jyoti A, Srinag BS, Keshari RS, Saluja R et al (2010) Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide 22(3):226–234
Awasthi D, Nagarkoti S, Kumar A, Dubey M, Singh AK, Pathak P et al (2016) Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radical Biol Med 93:190–203
Remijsen Q, Vanden T, Wirawan E, Asselbergh B, Parthoens E, De Rycke R et al (2011) Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res 21(2):290–304
Douda DN, Khan MA, Grasemann H, Palaniyar N (2015) SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci USA 112(9):2817–2822
Arai Y, Nishinaka Y, Arai T, Morita M, Mizugishi K, Adachi S et al (2014) Uric acid induces NADPH oxidase-independent neutrophil extracellular trap formation. Biochem Biophys Res Commun 443(2):556–561
Chen K, Nishi H, Travers R, Tsuboi N, Martinod K, Wagner DD et al (2012) Endocytosis of soluble immune complexes leads to their clearance by FcgammaRIIIB but induces neutrophil extracellular traps via FcgammaRIIA in vivo. Blood 120(22):4421–4431
Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD et al (2010) A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol 185(12):7413–7425
Ramirez-Weber FA, Kornberg TB (1999) Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 97(5):599–607
Hsiung F, Ramirez-Weber FA, Iwaki DD, Kornberg TB (2005) Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature 437(7058):560–563
Karp GC, Solursh M (1985) Dynamic activity of the filopodia of sea urchin embryonic cells and their role in directed migration of the primary mesenchyme in vitro. Dev Biol 112(2):276–283
Galkina SI, Fedorova NV, Serebryakova MV, Romanova JM, Golyshev SA, Stadnichuk VI et al (2012) Proteome analysis identified human neutrophil membrane tubulovesicular extensions (cytonemes, membrane tethers) as bactericide trafficking. Biochim Biophys Acta 1820(11):1705–1714
Galkina SI, Fedorova NV, Serebryakova MV, Arifulin EA, Stadnichuk VI, Gaponova TV et al (2015) Inhibition of the GTPase dynamin or actin depolymerisation initiates outward plasma membrane tubulation/vesiculation (cytoneme formation) in neutrophils. Biol Cell 107(5):144–158
Galkina SI, Fedorova NV, Stadnichuk VI, Sud’ina GF (2013) Membrane tubulovesicular extensions (cytonemes): secretory and adhesive cellular organelles. Cell Adh Migr 7(2):174–186
Galkina SI, Fedorova NV, Golenkina EA, Stadnichuk VI, Sud'ina GF (2020) Cytonemes versus neutrophil extracellular traps in the fight of neutrophils with microbes. Int J Mol Sci 21(2)
Galkina SI, Romanova JM, Stadnichuk VI, Molotkovsky JG, Sud’ina GF, Klein T (2009) Nitric oxide-induced membrane tubulovesicular extensions (cytonemes) of human neutrophils catch and hold Salmonella enterica serovar Typhimurium at a distance from the cell surface. FEMS Immunol Med Microbiol 56(2):162–171
Keshari RS, Jyoti A, Kumar S, Dubey M, Verma A, Srinag BS et al (2012) Neutrophil extracellular traps contain mitochondrial as well as nuclear DNA and exhibit inflammatory potential. Cytometry A 81(3):238–247
Keshari RS, Verma A, Barthwal MK, Dikshit M (2013) Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J Cell Biochem 114(3):532–540
Patel S, Kumar S, Jyoti A, Srinag BS, Keshari RS, Saluja R, et al (2010) Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide 22(3):226–34
Lim MB, Kuiper JW, Katchky A, Goldberg H, Glogauer M (2011) Rac2 is required for the formation of neutrophil extracellular traps. J Leukoc Biol 90(4):771–776
Das SK, Yuan YF, Li MQ (2018) Specific PKC betaII inhibitor: one stone two birds in the treatment of diabetic foot ulcers. Biosci Rep 38(5)
Zhao ML, Chi H, Sun L (2017) Neutrophil extracellular traps of cynoglossus semilaevis: production characteristics and antibacterial effect. Front Immunol 8:290
Manda-Handzlik A, Bystrzycka W, Cieloch A, Glodkowska-Mrowka E, Jankowska-Steifer E, Heropolitanska-Pliszka E, et al (2019) Nitric oxide and peroxynitrite trigger and enhance release of neutrophil extracellular traps. Cell Mol Life Sci (CMLS)
Galkina SI, Molotkovsky JG, Ullrich V, Sud'ina GF (2005) Scanning electron microscopy study of neutrophil membrane tubulovesicular extensions (cytonemes) and their role in anchoring, aggregation and phagocytosis: the effect of nitric oxide. Exp Cell Res 304(2):620–9
Urbano R, Karlinsey JE, Libby SJ, Doulias PT, Ischiropoulos H, Warheit-Niemi HI, et al (2018) Host nitric oxide disrupts microbial cell-to-cell communication to inhibit staphylococcal virulence. Cell Host Microbe 23(5):594–606 e7
Zagryazhskaya AN, Lindner SC, Grishina ZV, Galkina SI, Steinhilber D, Sud’ina GF (2010) Nitric oxide mediates distinct effects of various LPS chemotypes on phagocytosis and leukotriene synthesis in human neutrophils. Int J Biochem Cell Biol 42(6):921–931
Galkina SI, Fedorova NV, Serebryakova MV, Arifulin EA, Stadnichuk VI, Baratova LA et al (2017) Mold alkaloid cytochalasin D modifies the morphology and secretion of fMLP-, LPS-, or PMA-stimulated neutrophils upon adhesion to fibronectin. Mediators Inflamm 2017:4308684
Pryor WA, Squadrito GL (1995) The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol 268(5 Pt 1):L699-722
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87(1):315–424
Galkina SI, Sud’ina GF, Klein T (2006) Metabolic regulation of neutrophil spreading, membrane tubulovesicular extensions (cytonemes) formation and intracellular pH upon adhesion to fibronectin. Exp Cell Res 312(13):2568–2579
Li WX, Wang F, Zhu YQ, Zhang LM, Zhang ZH, Wang XM (2020) Inhibitors of nitric oxide synthase can reduce extracellular traps from neutrophils in asthmatic children in vitro. Pediatr Pulmonol 55(1):68–75
Ode Y, Aziz M, Wang P (2018) CIRP increases ICAM-1(+) phenotype of neutrophils exhibiting elevated iNOS and NETs in sepsis. J Leukoc Biol 103(4):693–707
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kumar, S., Sadaf, S., Dikshit, M. (2023). Role of Nitric Oxide Synthase and Nitric Oxide Signaling in the Neutrophil Ontogeny and Functions. In: Ray, A., Gulati, K. (eds) Nitric Oxide: From Research to Therapeutics. Advances in Biochemistry in Health and Disease, vol 22. Springer, Cham. https://doi.org/10.1007/978-3-031-24778-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-24778-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-24777-4
Online ISBN: 978-3-031-24778-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)