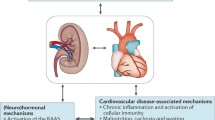
Abstract
There are numerous interactions between the cardiac and renal systems important to the management of heart failure. These interactions are bidirectional, in that acute or chronic dysfunction of either the heart or kidneys can then induce acute and chronic changes in the other organ as well. Mortality is increased in patients with heart failure who have reduced renal function. Likewise, cardiovascular disease is responsible for up to 50% of deaths in patients with renal failure. An incomplete understanding of the intricate partnership between these two organs can lead to limitations in heart failure guideline-directed medication therapy and worsening patient outcomes. Primary care serves as a hub for patient communication, collection of test results, and overall collaboration among specialty providers, including cardiology and nephrology making primary care providers a key link to the management of heart failure and cardiorenal disease. In this chapter, we will summarize the interactions between heart failure and renal dysfunction and the clinical management thereof focusing on both heart failure patients with cardiorenal syndrome and those with chronic renal insufficiency, ending in two separate case studies that highlight the bidirectional considerations necessary for the care of each group.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
National Heart, Lung, and Blood Institute. NHLBI Working Group: cardio-renal connections in heart failure and cardiovascular disease. 2004. https://www.nhlbi.nih.gov/events/2004/cardio-renal-connections-heart-failure-and-cardiovascular-disease. Accessed November 30, 2021.
Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, et al. Cardio-renal syndromes: report from the Consensus Conference of the Acute Dialysis Quality Initiative. Eur Heart J. 2010;31:703–11.
Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39. https://doi.org/10.1016/j.jacc.2008.07.051.
Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: A scientific statement from the American heart association. Circulation. 2019;139(16):e840–78. https://doi.org/10.1161/CIR.0000000000000664.
Beigel R, Cercek B, Siegel RJ, Hamilton MA. Echo-Doppler hemodynamics: an important management tool for today’s heart failure care. Circulation. 2015;131:1031–4. https://doi.org/10.1161/CIRCULATIONAHA.114.011424.
Kiernan M, Udelson J, Sarnak M. Cardiorenal syndrome: definition, prevalence, diagnosis, and pathophysiology. In: Gottlieb S, Dardas T, editors. Uptodate; 2022. [Cited 2022 Jan 13].
Mavrakanas TA, Khattak A, Singh K, Charytan DM. Epidemiology and natural history of the cardiorenal syndromes in a cohort with echocardiography. Clin J Am Soc Nephrol. 2017;12:1624–33. https://doi.org/10.2215/CJN.04020417.
Adams KF, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–16. https://doi.org/10.1016/j.ahj.2004.08.005.
Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–96. https://doi.org/10.1016/j.jacc.2008.05.068.
Mullens W, Abrahams Z, Skouri HN, Francis GS, Taylor DO, Starling RC, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol. 2008;51:300–6. https://doi.org/10.1016/j.jacc.2007.09.043.
Ichikawa I, Pfeffer JM, Pfeffer MA, Hostetter TH, Brenner BM. Role of angiotensin II in the altered renal function of congestive heart failure. Circ Res. 1984;55:669–75.
Ismail Y, Kasmikha Z, Green HL, McCullough PA. Cardio-renal syndrome type 1: epidemiology, pathophysiology, and treatment. Semin Nephrol. 2012;32(1):18–25. https://doi.org/10.1016/j.semnephrol.2011.11.003.
Maisel AS, Daniels LB, Anand IS, McCullough PA, Chow SL. Utility of natriuretic peptides to assess and manage patients with heart failure receiving angiotensin receptor blocker/neprilysin inhibitor therapy. Postgrad Med. 2018;130:299–307. https://doi.org/10.1080/00325481.2018.1440873.
Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–8. https://doi.org/10.1016/j.jacc.2008.08.080.
Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408. https://doi.org/10.1172/JCI46122.
Makris K, Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. 2016;37(2):85–98.
Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: Answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772–810. https://doi.org/10.1016/j.jacc.2020.11.022.
McCullough PA, Duc P, Omland T, McCord J, Nowak RM, Hollander JE, et al. Breathing Not Properly Multinational Study Investigators. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41:571–9. https://doi.org/10.1053/ajkd.2003.50118.
Gaião S, Cruz DN. Baseline creatinine to define acute kidney injury: is there any consensus? Nephrol Dial Transplant. 2010;25:3812–4.
Fried L. When increase in serum creatinine doesn’t imply kidney damage. Clin J Am Soc Nephrol. 2020;15(3):304–5. Available from https://cjasn.asnjournals.org/content/15/3/304.
Brater DC, Ellison DH. Causes and treatment of refractory edema in adults. In: Sterns RH, Emmett M, Forman JP, editors. Uptodate; 2022 [Cited 2022 Mar 16].
Park JH, Marwick TH. Use and limitations of E/e’ to assess left ventricular filling pressure by echocardiography. J Cardiovasc Ultrasound. 2011;19:169–73. https://doi.org/10.4250/jcu.2011.19.4.169.
Hassanin N, Alkemary A. Early detection of subclinical uremic cardiomyopathy using two-dimensional speckle tracking echocardiography. Echocardiography. 2016;33:527–36. https://doi.org/10.1111/echo.13120.
Faubel S, Patel NU, Lockhart ME, Cadnapaphornchai MA. Renal relevant radiology: use of ultrasonography in patients with AKI. Clin J Am Soc Nephrol. 2014;9:382–94. https://doi.org/10.2215/CJN.04840513.
Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T, et al. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail. 2016;4:674–82. https://doi.org/10.1016/j.jchf.2016.03.016.
Nijst P, Martens P, Dupont M, Tang WHW, Mullens W. Intrarenal flow alterations during transition from euvolemia to intravascular volume expansion in heart failure patients. JACC Heart Fail. 2017;5:672–81. https://doi.org/10.1016/j.jchf.2017.05.006.
Edwards NC, Moody WE, Chue CD, Ferro CJ, Townend JN, Steeds RP. Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2014;7:703–14. https://doi.org/10.1016/j.jcmg.2013.09.025.
Kiernan MS, Udelson JE, Sarnak M. Cardiorenal syndrome: prognosis and treatment. In: Gottlieb SS, Dardas TF. Uptodate; 2022 [Cited 2022 Mar 13].
Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–74. https://doi.org/10.1016/j.jacc.2007.08.072.
Colucci WS, Sterns RH. Use of diuretics in patients with heart failure. In: Gottlieb SS, Emmett M, Dardas TF, editors. Uptodate; 2022 (Cited 2022 Mar 13].
Sica DA. Pharmacotherapy in congestive heart failure: drug absorption in the management of congestive heart failure: loop diuretics. Congest Heart Fail. 2003;9:287–92.
House AA, Wanner C, Sarnak MJ, Piña IL, McIntyre CW, Komenda P, et al. Heart failure in chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2019;95(6):1304–17. Available from https://www.kidney-international.org/article/S0085-2538(19)30276-5/fulltext.
Rahman M, Ford CE, Cutler JA, et al. Long-term renal and cardiovascular outcomes in antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) participants by baseline estimated GFR. Clin J Am Soc Nephrol. 2012;7:989–1002.
Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9.
Wanner C, Lachin JM, Inzucchi SE, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018;137:119–29.
CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429–35.
Meyer TE. Initial pharmacologic therapy of heart failure with reduced ejection fraction in adults. In: Gottlieb SS, Dardas TF, editors. Uptodate; 2022 [Cited 2022 Mar 15].
Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R Jr, Ferdinand K, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351(20):2049–57. https://doi.org/10.1056/nejmoa042934.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17. https://doi.org/10.1056/NEJM199909023411001.
Ultrafiltration [Internet]. National Kidney Foundation. 2019 [cited 2022 Mar 16]. Available from https://www.kidney.org/atoz/content/ultrafiltration.
McCullough PA, Chan CT, Weinhandl ED, et al. Intensive hemodialysis, left ventricular hypertrophy, and cardiovascular disease. Am J Kidney Dis. 2016;68:S5–S14.
Schaffer JM, Chiu P, Singh SK, et al. Heart and combined heart-kidney transplantation in patients with concomitant renal insufficiency and end-stage heart failure. Am J Transplant. 2014;14:384–96.
Pitt B, Bakris GL, Bushinsky DA, et al. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail. 2015;17:1057–65.
Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223–33.
Colucci WS. Evaluation and management of anemia and iron deficiency in adults with heart failure. In: Gottlieb SS, Dardas TF, Tirnauer JS, editors. Uptodate; 2022 [Cited 2022 Mar 14].
Berns JS. Treatment of anemia in nondialysis chronic kidney disease. In: Golper TA, Forman JP, editors. Uptodate; 2022 [Cited 2022 Mar 13].
Ishani A, Weinhandl E, Zhao Z, Gilbertson DT, Collins AJ, Yusuf S, et al. Angiotensin-converting enzyme inhibitor as a risk factor for the development of anemia, and the impact of incident anemia on mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2005;45(3):391–9. Available from: https://pubmed.ncbi.nlm.nih.gov/15680718/.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendices
Case Study 1: Cardiorenal Syndrome Type II and Diuretic Resistance
Subjective: Ms. Jones is a 68 year-old female with the following past medical history/problem list: Heart failure with reduced ejection fraction, NYHA Class II; status post-ICD implant for primary prevention of sudden cardiac death; dilated, ischemic cardiomyopathy; coronary artery disease, status post-coronary artery bypass grafting >10 years ago; hypertension; diabetes mellitus, type 2.
Family history: Coronary artery disease in her father and paternal grandmother.
Social history: She lives at home with her husband. They have three grown children. Homemaker. Denies alcohol, tobacco, and illicit drug use.
Medications: Carvedilol 3.125 mg BID; Sacubitril/Valsartan 24/26 mg BID; Spironolactone 25 mg daily; Furosemide 80 mg BID; Metformin 1000 mg BID; Aspirin 81 mg daily; Atorvastatin 80 mg QHS.
Allergies: NKDA.
1.1 Case Scenario
Chief complaint: The patient called requesting an appointment due to worsening shortness of breath and her “fluid pill not working anymore.”
HPI: Ms. Smith returns for episodic visit complaining of increased shortness of breath with mild activity, 11 lb weight gain in 1 week, and lower extremity edema. She has recently returned from a vacation with her grandchildren where she admits she did not watch her sodium intake and ate out almost every day. She states, “I’ve been taking my furosemide, but it just doesn’t seem to be working as well as it used to.” She normally limits sodium intake to 2 g daily and fluid intake to 2 l daily and reports taking all medications as prescribed. She admits to bloating, early satiety, 3-pillow orthopnea, and less than expected urinary output. She denies any recent ER visits, hospitalizations, chest pain, or palpitations. No fever, dark or foul-smelling urine, frequency, urgency, frank hematuria, or hesitation.
1.2 Objective
Vital signs: BP 102/65; HR 89; oxygen saturation 97% on room air; Temp 98.2°. Weight 172 lbs (last recorded office weight was 161 lbs)
Physical exam: JVD 10 cm. Normal S, S2 without S3 or murmur. Normal work of breathing at rest. Lung sounds decreased in bilateral bases. Abdomen distended but still soft and non-tender. 1 + bilateral lower extremity pitting edema to mid-calves
1.3 Labs
Today—Sodium 134; Potassium 4.6; BUN 32; Creatinine 1.8; Pro BNP 3560
2 months ago—Sodium 137; Potassium 3.9; BUN 22; Creatinine 1.2; Pro BNP 540
1.4 Diagnostics
The most recent echo 2 months ago showed a stable EF of 35%.
1.5 Assessment
Ms. Jones is having a mild acute chronic heart failure exacerbation with NYHA Class III symptoms complicated by an acute decrease in renal function with a rise from baseline creatinine from 1.2 to now 1.8 over the last 2 months. Her pro-BNP is also elevated much higher than baseline. On exam, she appears to have increased abdominal pressure and congestion which is likely causing diuretic resistance to her furosemide and associated lab fluctuations.
1.6 Plan
Change furosemide 80 mg BID to bumetanide 4 mg in the morning and 2 mg in the afternoon for the next week starting today. Repeat visit with BMP in 5–7 days. Call in 1–2 days if urinary output does not increase with medication change or if shortness of breath or swelling continues to worsen.
Check weight daily upon waking after emptying the bladder and before eating or drinking. Call for further weight gain of 2 lbs overnight or weight loss greater than 10 lbs in 1 week.
Resume a low sodium diet and reduce fluid intake to 1.5 L/day until symptoms improve.
Return to the clinic for a recheck of symptoms in about 1 week. Would consider the addition of an SGLT2 inhibitor at the next visit for heart failure, diabetes, and renal protective benefits and decrease bumetanide to 2 mg BID (once the goal is reached) with an additional 2 mg PRN for a weight gain of 2 lbs overnight or 5 lbs in 1 week.
1.7 Clinical Pearls
-
Although this patient is experiencing acute symptoms of both cardiac and renal symptoms, she does not need to be treated as an inpatient or go to ER for IV diuretics unless oral medications do not help or symptoms worsen.
-
Changing furosemide to bumetanide should improve diuretic resistance and drug absorption. Increasing the dose temporarily will also be beneficial.
-
SGLT2 inhibitor benefits heart failure, diabetes, and renal function.
-
Lab monitoring expectations—creatinine will likely rise slightly at the next visit from increased diuretic use, but symptoms and BNP should improve. Renal function will then return to baseline over the next few weeks. Would trend labs every 2 weeks. If creatinine does not return to baseline in the next 1–2 months would consider a nephrology referral.
-
Monitor NT pro-BNP while taking sacubitril/valsartan.
Case Study 2: The Complex Interaction of CKD, HF, and Anemia
Subjective: Mr. Greene is a 55 year-old male with a past medical history/problem list: poorly controlled hypertension 20+ years; microscopic hematuria 10 years; nephrolithiasis; depression, obesity
Family history: Hypertension
Social history: Tool and dye maker. Divorced with 2 grown children. Denies current alcohol, tobacco, and illicit drug use. History of 1 ppd smoker for 30 years.
Medications: Lisinopril 20 mg daily, amlodipine 5 mg daily, hydrochlorothiazide 25 mg daily, sertraline 20 mg daily
Allergies: NKDA
1.1 Case Scenario
Chief complaint: Increased shortness of breath, swelling in ankles, and a metallic taste in the mouth for 2 months.
HPI: Since his last visit 9 months ago, the patient has noticed shortness of breath with walking short distances, swelling in the ankles, and a metallic taste in the mouth for about 2 months. He has checked his BP at home a couple of times and says it averages 150s/90s. He has also noted weight gain of about 15 lbs, bloating, and poor appetite. He states, “I just feel so tired all the time now.” He denies any missed doses of medications, lightheadedness, chest pain, or palpitations. No recent illness or hospitalizations.
1.2 Objective
Vital signs: BP 145/100; HR 102; oxygen saturation 96% on room air; Temp 98.0°. Weight 258 lbs
Physical exam: JVP elevated 12 cm; displaced apical beat (mid-axillary line); loud S3; lung fields clear, dull at both bases; liver edge 6 cm below costal margin; No ascites; 2+ Ankle edema
Labs: Serum creatinine 2.8 mg/dl; BUN 30; eGFR 26%; Potassium 4.0; Total CO2 28; Hemoglobin 10.3; (add diff showing anemia). Total cholesterol 216, LDL 146, triglycerides 362; fasting glucose 122; Albumin-creatinine ratio >300; Urinalysis 3+ protein, 5–10 rbc, No rbc cast–trace granular cast
No prior labs for comparison in the last 12 months.
Assessment: This patient has labs indicative of chronic renal insufficiency and anemia. He also has signs and symptoms of new-onset heart failure.
Plan: The patient needs an echocardiogram to better assess for LV dysfunction and referral to cardiology for management. He also needs a nephrology referral for CKD stage IV based on GFR. Would stop HCTZ and begin furosemide 80 mg BID for better diuresis. Would decrease lisinopril and begin hydralazine 100 mg TID for tighter BP control. Needs iron levels checked and replaced if indicated for anemia. Reduced sodium diet. A renal US for secondary hypertension workup and assessment of intrinsic kidney disease. Avoid nephrotoxins including NSAIDs.
1.3 Clinical Pearls
-
Diuresis is less effective with low-dose thiazide-like diuretics alone in the setting of CKD; he will need a higher dose loop diuretic to break the threshold and begin diuresis.
-
Current guidelines recommend reducing, not discontinuing ACE/ARB, with isolated elevated creatinine reading. Would add hydralazine for blood pressure coverage while further evaluation takes place. Would add isosorbide dinitrate if the echo reveals HFrEF.
-
Stabilization including appropriate diagnosis of renal disease, aggressive HTN management, and fluid volume likely to improve cardiac symptoms and quality of life.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Parker, M.M., Wigger, M. (2023). Cardiorenal Syndrome, Chronic Kidney Disease, Anemia, and Heart Failure. In: Hayes, K.M.S., Dellise, N.R. (eds) Managing Heart Failure in Primary Care: A Case Study Approach. Springer, Cham. https://doi.org/10.1007/978-3-031-20193-6_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-20193-6_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-20192-9
Online ISBN: 978-3-031-20193-6
eBook Packages: MedicineMedicine (R0)