Abstract
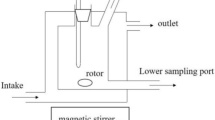
Due to the increase in energy consumption at the global level, it is necessary to examine new possibilities of substitution of classical energy-intensive separation processes based on evaporation of more volatile components, separation processes based on liquid-liquid equilibria (LLE), i.e. extraction of components from liquid solutions. This procedure would also include the replacement of standard industrial solvents with environmentally harmful characteristics with new, green solvents. Diethyl adipate can be used for these purposes because it is relatively non-toxic, obtained from renewable sources and is biodegradable. In order to examine the possibilities of using green solvents, the possibilities of adequate replacement and process design, it is necessary to know thermodynamic data such as liquid-liquid equilibria data, the corresponding binodal curve and equilibrium lines. The liquid-liquid equilibria data of the ternary system water + ethanol + diethyl adipate will be determined experimentally at a temperature of 298.15 K and atmospheric pressure. Binodal curves will be determined using the synthetic blur method using both the titration technique and the equilibrium lines via the refractive index. The obtained experimental data will be used in determining the complete phase diagram of the mentioned system.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Dai, Y., Zheng, F., **a, B., Cui, P., Wang, Y.: Jun Gao, Application of Mixed Solvent To Achieve an Energy-Saving Hybrid Process Including Liquid−Liquid Extraction and Heterogeneous Azeotropic Distillation. Ind. Eng. Chem. Res. 58, 2379–2388 (2019)
Malicoa, I., Pereiraa, R.N., Gonçalvesa, A.C., Sousa, A.M.O.: Current status and future perspectives for energy production from solid biomass in the European industry. Renew. Sustain. Energy Rev. 112, 960–977 (2019)
Baranidharan, M., Kanna, R.R., Singh, R.R.: Energy Saving on Industrial Drive Technologies - Past, Present, and Future Perspective, IOP Conf. Series: Materials Science and Engineering 906, 012005 (2020)
Giraud, R.J., Williams, R.A., Sehgal, A., Ponnusamy, E., Phillips, A.K., Manley, J.B.: Implementing green chemistry in chemical manufacturing: a survey report. ACS Sustainable Chem. Eng. 2237−2242 (2014)
Aljimaz, A., Fandary, M.S.H., Alkandary, J.A., Fahim, M.A.: Liquid-liquid equilibria of the ternary system water + acetic acid + 1-heptanol. J. Chem. Eng. Data 45, 301–303 (2000)
Nann, A., Held, C., Sadowski, G.: Liquid−liquid equilibria of 1-Butanol/Water/IL Systems. Ind. Eng. Chem. Res. 52, 18472–18481 (2013)
Garcıa-Flores, B.E., Galicia-Aguilar, G., Eustaquio-Rincón, R., Trejo, A.: Liquid–liquid phase diagrams of ternary systems as a function of temperature: isooctane + aromatic + methanol with and without water. Fluid Phase Equilib. 185, 275–293 (2001)
Wales, M.D., et al.: Liquid−liquid equilibria for ternary systems of water + methoxycyclopentane + alcohol (Methanol, Ethanol, 1-Propanol, or 2-Propanol). J. Chem. Eng. Data 61, 1479–1484 (2016)
Ghanadzadeh, H., Ghanadzadeh, A.: (Liquid + liquid) equilibria in (water + ethanol + 2-ethyl-1-hexanol) at T ¼ (298.2, 303.2, 308.2, and 313.2) K. J. Chem. Thermodynamics 35, 1393–1401 (2003)
Ivaniš, G.R., Vuksanović, J.M., Calado, M.S., Kijevčanin, M.L., Šerbanović, S.P., Višak, Z.P.: Liquid-liquid and solid-liquid equilibria in the solutions of poly(ethylene glycol) with several organic solvents. Fluid Phase Equilib. 316, 74–84 (2012)
Najdanović-Višak, V., et al.: Co-solvent effects in LLE of 1-hydroxyethyl-3-methylimidazolium based ionic liquids + 2-propanol + dichloromethane or 1,2-dichloroethane. Fluid Phase Equilib. 254, 35–41 (2007)
Ghanadzadeh, H., Ghanadzadeh, A.: Liquid–liquid phase equilibria of the ternary system of water/TBA/2-ethyl-1-hexanol. Fluid Phase Equilib. 202, 337–344 (2002)
Alkandary, J.A., Aljimaz, A.S., Fandary, M.S., Fahim, M.A.: Liquid–liquid equilibria of water + MTBE + reformate. Fluid Phase Equilib. 187–188, 131–138 (2001)
Logsdon, J.E.: Ethanol. Kirk-Othmer Encyclopedia of Chemical Technology. s.l. John Wiley & Sons Inc. (2000)
Oliveira, F.S., Pereiro, A.B., Rebeloa, L.P.N., Marrucho, I.M.: Deep eutectic solvents as extraction media. Green Chemistry Journal 15, 1326–1330 (2013)
Chaibakhsh, N., Abdul Rahman, M.N., Abu Bakar Salleh, M.B.: Rahman RNZR. Effect of alcohol chain length on the optimum conditions for lipase-catalyzed synthesis of adipate esters 27, 303–308 (2009)
Zhu, R., Cheung, C.S., Huang, Z., Wang, X.: Regulated and unregulated emissions from a diesel engine fueled with diesel fuel blended with diethyl adipate. Athmospheric Enviroment Jurnal 45, 2174–2181 (2011)
Cho, J., Park, J., Jeon, J.: Comparison of three- and two-column configurations in ethanol dehydration using azeotropic distillation. J. Ind. Eng. Chem. 12, 206–215 (2006)
Rodríguez, N.R., González, A.S.B., Tijssen, P.M.A., Kroon, M.C.: Low transition temperature mixtures (LTTMs) as novel entrainers in extractive distillation. Fluid Phase Equilib. 385, 72–78 (2015)
Anastas, P.T., Warner, J.C.: Green Chemistry: Theory and Practice. Oxford University Press, New York (2000)
Aissa, M., Radović, I., Simić, Z., Kijevcanin, K.: Thermodynamic and transport properties of ternary mixture (ethyl oleate + n-hexadecane + 1-butanol) and its binary constituents (ethyl oleate + 1-butanol and ethyl oleate + n-hexadecane) at different temperatures and atmospheric pressure. J. Mol. Liq. 317, 114186 (2020)
Simić, Z., Kijevčanin, M., Radović, I., Grilc, M., Gorica, I.: Thermodynamic and transport properties of biomass-derived furfural, furfuryl alcohol and their mixtures. Energies 14(22), 7769 (2021)
Ilić Pajić, J., et al.: Experimental densities and derived thermodynamic properties of pure p-cymene, α-pinene, limonene and citral under high pressure conditions. J. Chem. Thermodynamics 144, 106064 (2020)
Vuksanović, J., Soldatović, D., Radović, I., Višak, Z., Kijevčanin, M.: Thermodynamic characterization of binary mixtures of poly(propylene glycol) 425 with toluene and o-, m- and p-xylenes. J. Chem. Thermodynamics 131, 393–403 (2019)
Vuksanović, J., Kijevčanin, M.L., Radović, I.R.: Effect of water addition on extraction ability of eutectic solvent choline chloride+ 1,2-propanediol for separation of hexane/heptane+ethanol systems. Korean J. Chem. Eng. 35(7), 1477–1487 (2018). https://doi.org/10.1007/s11814-018-0030-z
Aissa, M., Radović, I., Kijevčanin, M.: A systematic study on volumetric and transport properties of binary systems 1-propanol + n-hexadecane, 1-butanol + n-hexadecane and 1-propanol + ethyl oleate at different temperatures: Experimental and modeling. Fluid Phase Equilib. 473, 1–16 (2018)
Vuksanović, J., Živković, E., Radović, I., Djordjević, B., Šerbanović, S., Kijevčanin, M.: Experimental study and modelling of volumetric properties, viscosities and refractive indices of binary liquid mixtures benzene+PEG 200/PEG 400 and toluene+PEG 200/PEG 400. Fluid Phase Equilib. 345, 28–44 (2013)
Ivanis, G., Vuksanovic, J., Calado, M., Kijevcanin, M., Serbanovic, S., Visak, Z.: Liquid-liquid and solid-liquid equilibria in the solutions of poly(ethylene glycol) with several organic solvents. Fluid Phase Equilib. 316, 74–84 (2012)
Knežević-Stevanović, A., Radović, I., Šerbanović, S., Kijevčanin, M.: Densities, viscosities, and refractive indices of the ternary mixture dimethyladipate + 2-butanone + 1-butanol at T = (288.15 to 323.15) K. Journal of Chemical and Engineering Data 59, 4133–4150 (2014)
Radović, I.: doctoral dissertation, Faculty of Technology and Metallurgy. University of Belgrade (2008)
Gilani, A.G., Gilani, H.G., Saadat, S.L.S., Nasiri-Touli, E., Peer, M.: Liquid-liquid equilibrium data in aqueous solutions of propionic and butyric acids with 1-heptanol at T=(298.15, 308.15, and 318.15) K. Korean J. Chem. Eng. 33, 1408–1415 (2016)
Lee, J.Y., Park, Y.: Usage of a deep eutectic solvent based on three compounds for toluene separation. Korean J. Chem. Eng. 35(1), 210–213 (2018). https://doi.org/10.1007/s11814-017-0244-5
Tang, W., Liu, L., Li, G., Zhu, T., Row, K.H.: Optimal separation of phenol from model oils by forming deep eutectic solvents with quaternary ammonium salts. Korean J. Chem. Eng. 34(3), 814–821 (2017). https://doi.org/10.1007/s11814-016-0316-y
Oliveira, F.S., Pereiro, A.B., Rebelo, L.P.N., Marrucho, I.M.: Deep eutectic solvents as extraction media for azeotropic mixtures. Green Chem. 15, 1326 (2013)
Simić, Z., Kijevčanin, M., Radović, I.: Liquid-liquid equilibrium of the ternary water solutions system in separation processes, 34. Procesing, Novi Sad, Serbia, June 3-4.2021, Book of abstract, 141–144, ISBN: 978-86-85535-08-6
Acknowledgment
The authors gratefully acknowledge the financial support received from the Research Fund of Ministry of Education, Science and Technological of the Republic of Serbia Development (Contract No. 451–03-68/2022–14/200135)), the Faculty of Technology and Metallurgy, University of Belgrade.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Simić, Z., Radović, I., Kijevčanin, M. (2023). Experimental Determination of Liquid-Liquid Equilibrium Data on Ternary Water Systems. In: Mitrovic, N., Mladenovic, G., Mitrovic, A. (eds) Experimental Research and Numerical Simulation in Applied Sciences. CNNTech 2022. Lecture Notes in Networks and Systems, vol 564. Springer, Cham. https://doi.org/10.1007/978-3-031-19499-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-19499-3_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-19498-6
Online ISBN: 978-3-031-19499-3
eBook Packages: Intelligent Technologies and RoboticsIntelligent Technologies and Robotics (R0)