Abstract.
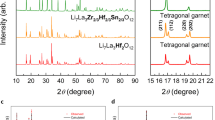
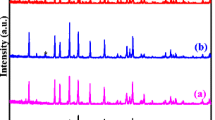
All-solid-state lithium-ion batteries (ASSLIBs) are considered to have substantial benefits over conventional Li-ion batteries based on flammable organic solvent electrolytes, such as high energy density, superior safety, and excellent mechanical strength [1–2]. The two types of battery architecture are compared in Fig. 1. As the core component of ASSLIBs, a lot of solid-state Li-ion conductors (electrolytes) have been developed and extensively explored in recent years, including crystalline Li1 + xAlxTi2 − x(PO4)3 (NASICON), Li7La3Zr2O12 (Garnet), Li3xLa2/3 − xTiO3 (Perovskite), Li10GeP2S12 (thio-LISICON), amorphous LiPON, etc [3–4]. Among them, Al-doped cubic-structured garnet-type Li7La3Zr2O12 (Al-c-LLZO) solid-state Li-ion conductor has attracted the most attention due to its relatively high lithium-ion conductivity (10−3 ~ 10−4 S cm−1 at ambient temperature), wide electrochemical window up to 6 V (vs. Li / Li+), and excellent chemical stability against lithium metal used as anode [5]. Despite of its good electrochemical properties, the commercial application potential of Al-c-LLZO is unfortunately hindered by its complex synthesis procedure and fabrication, which are time and energy consuming but also its interfacing with the electrodes. Up to now, the synthesis and processing of cubic-structured garnet conductors are based on sol-gel and solid-state synthesis and sintering usually done at temperatures above 1100 °C, as well as multiple cycles of sintering and milling [5]. This process results in micro-sized particles and potential Li-loss, which are unfavorable for further processing and electrode–electrolyte assembly with high Li-ion conductivity [6]. Here, we tackle this problem and firstly report a novel low-temperature synthesis-processing route to stabilize the cubic phase of Li6.1Al0.3La3Zr2O12 (Al-c-LLZO), while limiting particle growth to ~100–200 nm. This synthesis route involves three steps: (1) co-precipitation, (2) hydrothermal aging, and (3) calcination. The cubic Al-c-LLZO phase is obtained at temperatures as low as 600 °C by reactive transformation/calcination of aqueous co-precipitated metal hydroxide intermediate of controlled crystal orientation. Through extensive structural characterization of the samples collected after different synthesis steps, the crystalline phase transition from M(OH)x (amorphous) to M(OH)x (crystalline) to LazZryOw and finally to Li7-vAlv/3La3Zr2O12 (Al-c-LLZO) was observed and presented in Fig. 1. Based on the XRD patterns, the crystallization of Al-c-LLZO phase is fully developed when temperature reached 600 °C, and no further phase transition occurred when the temperature was raised to 700 °C, which suggests that the pure Al-c-LLZO phase can be obtained at 600 °C, the lowest calcination temperature compared to the published results so far. Moreover, according to the XRD results, the size of the primary nanocrystals of the low-T synthesized Al-c-LLZO was calculated through Scherrer equation to be ~29 nm. The morphology and particle size distribution of the low-T synthesized Al-c-LLZO particles are presented in Fig. 2. The mean size of the particles is 180 nm. As for the Li-ion conductivity, the low-T synthesized Al-c-LLZO particles after one-step pellet compaction and sintering were characterized by Electrochemical Impedance Spectroscopy (EIS) at room temperature (21 °C) as well as from 30 to 80 °C with 10 °C temperature intervals. The Nyquist plots are shown in Fig. 3. The Li-ion conductivity at RT is approaching 10-3 S ∙ cm−1 and the calculated activation energy of Li-ion migration is 0.174 eV. Through this novel synthesis route, nanoscale Al-c-LLZO particles with high ionic conductivity are particularly attractive in promoting compatibility between cathode materials and solid electrolytes for ASSLIBs. The mechanism of the low-T synthesis route, as well as the results in detail, will be provided during the oral presentation (Fig. 4).
Senhao Wang is the presenting author of this chapter.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Janek J, Zeier WG. A solid future for battery development. Nat Energy. 2016;1(9):16141.
Masias A, Marcicki J, Paxton WA. Opportunities and challenges of lithium ion batteries in automotive applications. ACS Energy Lett. 2021;6(2):621–30.
Zhang B, Tan R, Yang L, Zheng J, Zhang K, Mo S, Lin Z, Pan F. Mechanisms and properties of ion-transport in inorganic solid electrolytes. Energy Stor Mater. 2018;10:139–59.
Murali A, Sakar M, Priya S, Vijayavarman V, Pandey S, Sai R, Katayama Y, Abdul Kader M, Ramanujam K. Insights into the emerging alternative polymer-based electrolytes for all solid-state lithium-ion batteries: a review. Mater Lett. 2022;313:131764.
Xu L, Li J, Deng W, et al. Garnet solid electrolyte for advanced all‐solid‐state Li batteries[J]. Advanced Energy Materials, 2021, 11(2): 2000648.
Balaish M, Gonzalez-Rosillo JC, Kim KJ, Zhu Y, Hood ZD, Rupp JLM. Processing thin but robust electrolytes for solid-state batteries. Nat Energy. 2021;6(3):227–39.
Acknowledgments
This work is supported by NSERC, Hydro-Québec, and McGill’s Engineering Doctoral Award program.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Wang, S., Chiu, Hc., Demopoulos, G.P. (2023). Crystallization of Nanoscale Garnet-Type Li-Ion Conductors for All-Solid-State Batteries (ASSBs). In: Proceedings of the 61st Conference of Metallurgists, COM 2022. COM 2022. Springer, Cham. https://doi.org/10.1007/978-3-031-17425-4_36
Download citation
DOI: https://doi.org/10.1007/978-3-031-17425-4_36
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-17424-7
Online ISBN: 978-3-031-17425-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)