Summary
Tuberculosis (TB) is a dangerous infectious disease caused by Mycobacterium tuberculosis (M. tb). Although approximately 90% of individuals are infected with M. tb, the disease develops only in 5–10% of the infected people. Other persons infected with M. tb remain healthy during their entire life, i.e., it can be assumed that the symbiotic host–pathogen interactions emerge to prevent not only TB but also other diseases. We believe that the host immune system is important in develo** TB-associated inflammation and another response of this system to M. tb significantly contributes to TB development. Modulation of immunity via immunotherapeutic agents may balance host–pathogen interactions. Conventional chemotherapeutic anti-TB drugs aim at eliminating M. tb, whereas the imbalance in the immunopathological process should be modified by immunotherapy. Adjunctive immunotherapy may enhance the treatment effectiveness, reduce the duration of chemotherapy, and improve the host immunity preventing TB relapses. This chapter deals with the most common types of immunotherapeutic strategies that have been used in clinical trials.
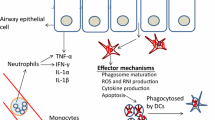
Graphical Abstract

Immunotherapy for tuberculosis (TB)
If you change the way you look at things, the things you look at change.
Max Planck
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Global Tuberculosis Report 2019: WHO Report 2021 (2021) World Health Organization. https://www.who.int/publications/i/item/9789240037021
The End TB Strategy (2015) Global strategy and targets for tuberculosis prevention, care and control after 2015. World Health Organisation. https://www.who.int/tb/post2015_TBstrategy.pdf
Dudnyk A, Butov D, Crudu V, Lange C, Chesov D (2017) MDR-TB in Eastern Europe in the era of the TB elimination action framework. Int J Tuberc Lung Dis 21:2–3
Butov D, Myasoedov V, Gumeniuk M, Gumeniuk G, Choporova O, Tkachenko A, Akymenko O, Borysova O, Goptsii O, Ye V, Butova T (2020) Treatment effectiveness and outcome in patients with a relapse and newly diagnosed multidrug-resistant pulmonary tuberculosis. Med Glas (Zenica) 17(2):356–362. https://doi.org/10.17392/1179-20
WHO announces updated definitions of extensively drug-resistant tuberculosis (2021) World Health Organisation. https://www.who.int/news/item/27-01-2021-who-announces-updated-definitions-of-extensively-drug-resistant-tuberculosis
World Health Organisation consolidated guidelines on drug-resistant tuberculosis treatment (2019) World Health Organisation. https://apps.who.int/iris/bitstream/handle/10665/311389/9789241550529-eng.pdf?ua=1
Butov D, Lange C, Heyckendorf J, Kalmykova I, Butova T, Borovok N, Novokhatskaya M, Chesov D (2020) Multidrug-resistant tuberculosis in the Kharkiv Region, Ukraine. Int J Tuberc Lung Dis 24(5):485–491
Yew WW, Lange C, Leung CC (2011) Treatment of tuberculosis: update 2010. Eur Respir J 37(2):441–462
Nguyen TVA, Anthony RM, Bañuls AL, Nguyen TVA, Vu DH, Alffenaar JC (2018) Bedaquiline resistance: its emergence, mechanism, and prevention. Clin Infect Dis 66(10):1625–1630
Fujiwara M, Kawasaki M, Hariguchi N, Liu Y, Matsumoto M (2018) Mechanisms of resistance to delamanid, a drug for Mycobacterium tuberculosis [published correction appears in Tuberculosis (Edinb). 2018 Mar 31]. Tuberculosis (Edinb) 108:186–194
Olaru ID, von Groote-Bidlingmaier F, Heyckendorf J, Yew WW, Lange C, Chang KC (2015) Novel drugs against tuberculosis: a clinician’s perspective. Eur Respir J 45(4):1119–1131
Zaitzeva SI, Matveeva SL, Gerasimova TG, Pashkov YN, Butov DA, Pylypchuk VS, Frolov VM, Kutsyna GA (2019) Treatment of cavitary and infiltrating pulmonary tuberculosis with and without the immunomodulator Dzherelo. Clin Microbiol Infect 15(12):1154–1162
Butov DA, Efremenko YV, Prihoda ND, Yurchenko LI, Sokolenko NI, Arjanova OV, Stepanenko AL, Butova TS, Zaitzeva SS, Jirathitikal V, Bourinbaiar AS, Kutsyna GA (2012) Adjunct immune therapy of first-diagnosed TB, relapsed TB, treatment-failed TB, multidrug-resistant TB and TB/HIV. Immunotherapy 4(7):687–695
Butov D, Gumenuik M, Gumeniuk G, Tkachenko A, Kikinchuk V, Stepaniuk R, Peshenko A, Butova T (2019) Effectiveness of anti-tuberculosis chemotherapy in patients with tuberculosis relapse compared with newly diagnosed patients. Int J Mycobacteriol 8(4):341–346
Adepoju P (2020) Tuberculosis and HIV responses threatened by COVID-19. Lancet HIV 7(5):e319–e320
Bock P, Jennings K, Vermaak R, Cox H, Meintjes G, Fatti G, Kruger J, De Azevedo V, Maschilla L, Louis F, Gunst C, Grobbelaar N, Dunbar R, Limbada M, Floyd S, Grimwood A, Ayles H, Hayes R, Fidler S, Beyers N (2018) Incidence of tuberculosis among HIV-positive individuals initiating antiretroviral treatment at higher CD4 counts in the HPTN 071 (PopART) trial in South Africa. J Acquir Immune Defic Syndr 77(1):93–101
Abel L, El-Baghdadi J, Bousfiha AA, Casanova JL, Schurr E (2014) Human genetics of tuberculosis: a long and winding road. Philos Trans R Soc Lond B Biol Sci 369(1645):20130428
Butov DO, Kuzhko MM, Makeeva NI, Butova TS, Stepanenko HL, Dudnyk AB (2016) Association of interleukins genes polymorphisms with multi-drug resistant tuberculosis in Ukrainian population. Pneumonol Alergol Pol 84(3):168–173
de Martino M, Lodi L, Galli L, Chiappini E (2019) Immune response to Mycobacterium tuberculosis: a narrative review. Front Pediatr 7:350. https://doi.org/10.3389/fped.2019.00350 [Published online 2019 Aug 27]
Abate G, Hoft DF (2016) Immunotherapy for tuberculosis: future prospects. Immunotargets Ther 5:37–45. https://doi.org/10.2147/ITT.S81892
Li Y, Wang Y, Liu X (2012) The role of airway epithelial cells in response to mycobacteria infection. Clin Dev Immunol 2012:791392. https://doi.org/10.1155/2012/791392
Carranza C, Chavez-Galan L (2019) Several routes to the same destination: inhibition of phagosome-lysosome fusion by Mycobacterium tuberculosis. Am J Med Sci 357(3):184–194. https://doi.org/10.1016/j.amjms.2018.12.003
Jamwal SV, Mehrotra P, Singh A, Siddiqui Z, Basu A, Rao KV (2016) Mycobacterial escape from macrophage phagosomes to the cytoplasm represents an alternate adaptation mechanism. Sci Rep 6:23089. https://doi.org/10.1038/srep23089
Upadhyay S, Mittal E, Philips JA (2018) Tuberculosis and the art of macrophage manipulation. Pathog Dis 76(4):fty037. https://doi.org/10.1093/femspd/fty037
Stamm CE, Collins AC, Shiloh MU (2015) Sensing of Mycobacterium tuberculosis and consequences to both host and bacillus. Immunol Rev 264(1):204–219. https://doi.org/10.1111/imr.12263
Wong KW (2017) The role of ESX-1 in Mycobacterium tuberculosis pathogenesis. Microbiol Spectr 5(3): https://doi.org/10.1128/microbiolspec.TBTB2-0001-2015
Wawrocki S, Druszczynska M (2017) Inflammasomes in Mycobacterium tuberculosis-driven immunity. Can J Infect Dis Med Microbiol 2017:2309478. https://doi.org/10.1155/2017/2309478
Pesu M (2016) New insights into the host cell necrosis in tuberculosis. Virulence 7(1):1–2. https://doi.org/10.1080/21505594.2015.1122167
Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, Vance RE, Stallings CL, Virgin HW, Cox JS (2015) The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe 17(6):811–819. https://doi.org/10.1016/j.chom.2015.05.004
Hossain MM, Norazmi MN (2013) Pattern recognition receptors and cytokines in Mycobacterium tuberculosis infection—the double-edged sword? Biomed Res Int 2013:179174. https://doi.org/10.1155/2013/179174
Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R (2011) Innate immune recognition of Mycobacterium tuberculosis. Clin Dev Immunol 2011:405310. https://doi.org/10.1155/2011/405310
Zhou Y, Zhang M (2020) Associations between genetic polymorphisms of TLRs and susceptibility to tuberculosis: a meta-analysis. Innate Immun 26(2):75–83. https://doi.org/10.1177/1753425919862354
Wang C, Chen ZL, Pan ZF, Wei LL, Xu DD, Jiang TT, Zhang X, ** ZP, Li ZJ, Li JC (2013) NOD2 polymorphisms and pulmonary tuberculosis susceptibility: a systematic review and meta-analysis. Int J Biol Sci 10(1):103–108. https://doi.org/10.7150/ijbs.7585
Faridgohar M, Nikoueinejad H (2017) New findings of Toll-like receptors involved in Mycobacterium tuberculosis infection. Pathog Glob Health 111(5):256–264. https://doi.org/10.1080/20477724.2017.1351080
Biyikli OO, Baysak A, Ece G, Oz AT, Ozhan MH, Berdeli A (2016) Role of toll-like receptors in tuberculosis infection. Jundishapur J Microbiol 9(10):e20224. https://doi.org/10.5812/jjm.20224
Maceda EB, Gonçalves CCM, Andrews JR, Ko AI, Yeckel CW, Croda J (2018) Serum vitamin D levels and risk of prevalent tuberculosis, incident tuberculosis and tuberculin skin test conversion among prisoners. Sci Rep 8(1):997. https://doi.org/10.1038/s41598-018-19589-3
Negroni A, Pierdomenico M, Cucchiara S, Stronati L (2018) NOD2 and inflammation: current insights. J Inflamm Res 11:49–60. https://doi.org/10.2147/JIR.S137606
Brooks MN, Rajaram MV, Azad AK, Amer AO, Valdivia-Arenas MA, Park JH, Núñez G, Schlesinger LS (2011) NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell Microbiol 13(3):402–418. https://doi.org/10.1111/j.1462-5822.2010.01544.x
Paik S, Kim JK, Chung C, Jo EK (2019) Autophagy: a new strategy for host-directed therapy of tuberculosis. Virulence 10(1):448–459. https://doi.org/10.1080/21505594.2018.1536598
Bento CF, Empadinhas N, Mendes V (2015) Autophagy in the fight against tuberculosis. DNA Cell Biol 34(4):228–242. https://doi.org/10.1089/dna.2014.2745
Zhang R, ** X, Wang C, Pan Y, Ge C, Zhang L, Zhang S, Liu H (2018) Therapeutic effects of recombinant human interleukin 2 as adjunctive immunotherapy against tuberculosis: a systematic review and meta-analysis. PLoS ONE 13(7):e0201025. https://doi.org/10.1371/journal.pone.0201025
Liu X, Li F, Niu H, Ma L, Chen J, Zhang Y, Peng L, Gan C, Ma X, Zhu B (2019) IL-2 restores T-cell dysfunction induced by persistent Mycobacterium tuberculosis antigen stimulation. Front Immunol 10:2350. https://doi.org/10.3389/fimmu.2019.02350
Waters RS, Perry JSA, Han S, Bielekova B, Gedeon T (2018) The effects of interleukin-2 on immune response regulation. Math Med Biol 35(1):79–119. https://doi.org/10.1093/imammb/dqw021
Jacobs A, Wilkinson RJ (2015) Humoral immunity in tuberculosis [published correction appears in Eur J Immunol. 2015 Apr;45(4):1274]. Eur J Immunol 45(3):647–649. https://doi.org/10.1002/eji.201570034
Dannenberg AM Jr, Collins FM (2001) Progressive pulmonary tuberculosis is not due to increasing numbers of viable bacilli in rabbits, mice and guinea pigs, but is due to a continuous host response to mycobacterial products. Tuberculosis (Edinb) 81(3):229–242
Mootoo A, Stylianou E, Arias MA, Reljic R (2009) TNF-alpha in tuberculosis: a cytokine with a split personality. Inflamm Allergy Drug Targets 8(1):53–62. https://doi.org/10.2174/187152809787582543
Ravimohan S, Kornfeld H, Weissman D, Bisson GP (2018) Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev 27(147):170077. https://doi.org/10.1183/16000617.0077-2017
Bourinbaiar AS, Mezentseva MV, Butov DA, Nyasulu PS, Efremenko YV, Jirathitikal V, Mishchenko VV, Kutsyna GA (2012) Immune approaches in tuberculosis therapy: a brief overview. Expert Rev Anti Infect Ther 10(3):381–389
Butov D, Zaitseva S, Butova T, Stepanenko G, Pogorelova O, Zhelezniakova N (2016) Efficacy and safety of quercetin and polyvinylpyrrolidone in treatment of patients with newly diagnosed destructive pulmonary tuberculosis in comparison with standard antimycobacterial therapy. Int J Mycobacteriol 5(4):446–453
Zeng G, Zhang G, Chen X (2018) Th1 cytokines, true functional signatures for protective immunity against TB? Cell Mol Immunol 15(3):206–215. https://doi.org/10.1038/cmi.2017.113
Amaral EP, Lasunskaia EB, D’Império-Lima MR (2016) Innate immunity in tuberculosis: how the sensing of mycobacteria and tissue damage modulates macrophage death. Microbes Infect 18(1):11–20. https://doi.org/10.1016/j.micinf.2015.09.005
Young C, Walzl G, Du Plessis N (2020) Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol 13(2):190–204. https://doi.org/10.1038/s41385-019-0226-5
Zhang Z, Fan W, Yang G, Xu Z, Wang J, Cheng Q, Yu M (2017) Risk of tuberculosis in patients treated with TNF-α antagonists: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 7(3):e012567. https://doi.org/10.1136/bmjopen-2016-012567 [Published online 2017 Mar 22]
Olsen A, Chen Y, Ji Q, Zhu G, De Silva AD, Vilchèze C, Weisbrod T, Li W, Xu J, Larsen M, Zhang J, Porcelli SA, Jacobs WR Jr, Chan J (2016) Targeting Mycobacterium tuberculosis tumor necrosis factor alpha-downregulating genes for the development of antituberculous vaccines. mBio 7(3):e01023-15. https://doi.org/10.1128/mBio.01023-15.
Esmail H, Wilkinson RJ (2017) Minimizing tuberculosis risk in patients receiving anti-TNF therapy. Ann Am Thorac Soc 14(5):621–623. https://doi.org/10.1513/AnnalsATS.201701-055ED
Denis M, Ghadirian E (1993) Immunotherapy of airborne tuberculosis in mice via the lung-specific delivery of cytokines. Can J Infect Dis 4(1):38–42. https://doi.org/10.1155/1993/954372
Nolt D, Flynn JL (2004) Interleukin-12 therapy reduces the number of immune cells and pathology in lungs of mice infected with Mycobacterium tuberculosis. Infect Immun 72(5):2976–2988. https://doi.org/10.1128/iai.72.5.2976-2988.2004
Bermudez LE, Stevens P, Kolonoski P, Wu M, Young LS (1989) Treatment of disseminated Mycobacterium avium complex infection in mice with recombinant interleukin-2 and tumor necrosis factor. J Immunol 143:2996–3002
Bermudez LE, Young LS (1988) Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol 9:3006–3013
Skerry C, Harper J, Klunk M, Bishai WR, Jain SK (2012) Adjunctive TNF inhibition with standard treatment enhances bacterial clearance in a murine model of necrotic TB granulomas. PLoS ONE 7(6):e39680. https://doi.org/10.1371/journal.pone.0039680
Nikitina IY, Panteleev AV, Sosunova EV, Karpina NL, Bagdasarian TR, Burmistrova IA, Andreevskaya SN, Chernousova LN, Vasilyeva IA, Lyadova IV (2016) Antigen-specific IFN-γ responses correlate with the activity of M. tuberculosis infection but are not associated with the severity of tuberculosis disease. J Immunol Res 2016:7249369. https://doi.org/10.1155/2016/7249369
Sakai S, Kauffman KD, Sallin MA, Sharpe AH, Young HA, Ganusov VV, Barber DL (2016) CD4 T cell-derived IFN-γ plays a minimal role in control of pulmonary Mycobacterium tuberculosis infection and must be actively repressed by PD-1 to prevent lethal disease. PLoS Pathog 12(5):e1005667. https://doi.org/10.1371/journal.ppat.1005667
Tan Q, Min R, Dai GQ, Wang YL, Nan L, Yang Z, **a J, Pan SY, Mao H, **e WP, Wang H (2017) Clinical and immunological effects of rhIL-2 therapy in Eastern Chinese patients with multidrug-resistant tuberculosis. Sci Rep 7(1):17854. https://doi.org/10.1038/s41598-017-18200-5
Johnson BJ, Bekker LG, Rickman R, Brown S, Lesser M, Ress S, Willcox P, Steyn L, Kaplan G (1997) rhuIL-2 adjunctive therapy in multidrug resistant tuberculosis: a comparison of two treatment regimens and placebo. Tuber Lung Dis 78(3–4):195–203. https://doi.org/10.1016/s0962-8479(97)90026-5
Johnson JL, Ssekasanvu E, Okwera A, Mayanja H, Hirsch CS, Nakibali JG, Jankus DD, Eisenach KD, Boom WH, Ellner JJ, Mugerwa RD, Uganda-Case Western Reserve University Research Collaboration (2003) Randomized trial of adjunctive interleukin-2 in adults with pulmonary tuberculosis. Am J Respir Crit Care Med 168(2):185–191. https://doi.org/10.1164/rccm.200211-1359OC
Khan TA, Mazhar H, Saleha S, Tipu HN, Muhammad N, Abbas MN (2016) Interferon-gamma improves macrophages function against M. tuberculosis in multidrug-resistant tuberculosis patients. Chemother Res Pract 2016:7295390. https://doi.org/10.1155/2016/7295390
Gao XF, Yang ZW, Li J (2011) Adjunctive therapy with interferon-gamma for the treatment of pulmonary tuberculosis: a systematic review. Int J Infect Dis 15(9):e594–e600. https://doi.org/10.1016/j.ijid.2011.05.002
Suárez-Méndez R, García-García I, Fernández-Olivera N, Valdés-Quintana M, Milanés-Virelles MT, Carbonell D, Machado-Molina D, Valenzuela-Silva CM, López-Saura PA (2004) Adjuvant interferon gamma in patients with drug-resistant pulmonary tuberculosis: a pilot study. BMC Infect Dis 4:44. https://doi.org/10.1186/1471-2334-4-44
Zhang YQ, He CW, Li HQ, Zhao HL, Li BJ (2009) Effects of aerosolized interferon-γ in previously treated patients with smear-positive pulmonary tuberculosis. Med Recapitulate 15:306–307
Li D, Du DB, Qiu SQ, Meng QH, Luo SZ, Yuan RJ (2008) The early effectiveness and cellular immune function of interferon-γ combined with Mycobacterium vaccae for previously untreated pulmonary tuberculosis. J Chinese Anti-tuberc Assoc 30:460–461
Wallis RS, Kyambadde P, Johnson JL, Horter L, Kittle R, Pohle M, Ducar C, Millard M, Mayanja-Kizza H, Whalen C, Okwera A (2004) A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. AIDS 18(2):257–264. https://doi.org/10.1097/00002030-200401230-00015
Bourigault ML, Vacher R, Rose S, Olleros ML, Janssens JP, Quesniaux VF, Garcia I (2013) Tumor necrosis factor neutralization combined with chemotherapy enhances Mycobacterium tuberculosis clearance and reduces lung pathology. Am J Clin Exp Immunol 2(1):124–134
Vilaplana C, Cardona PJ (2010) Tuberculin immunotherapy: its history and lessons to be learned. Microbes Infect 12(2):99–105
Chahar M, Rawat KD, Reddy PVJ, Gupta UD, Natrajan M, Chauhan DS, Katoch K, Prasad GBKS, Katoch VM (2018) Potential of adjunctive Mycobacterium w (MIP) immunotherapy in reducing the duration of standard chemotherapy against tuberculosis. Indian J Tuberc 65(4):335–344
Butov DA, Efremenko YV, Prihoda ND, Zaitzeva SI, Yurchenko LV, Sokolenko NI, Butova TS, Stepanenko AL, Kutsyna GA, Jirathitikal V, Bourinbaiar AS (2013) Randomized, placebo-controlled Phase II trial of heat-killed Mycobacterium vaccae (Immodulon batch) formulated as an oral pill (V7). Immunotherapy 5(10):1047–1054
Yang XY, Chen QF, Li YP, Wu SM (2011) Mycobacterium vaccae as adjuvant therapy to anti-tuberculosis chemotherapy in never-treated tuberculosis patients: a meta-analysis. PLoS ONE 6(9):e23826
Bourinbaiar AS, Batbold U, Efremenko Y, Sanjagdorj M, Butov D, Damdinpurev N, Grinishina E, Mijiddorj O, Kovolev M, Baasanjav K, Butova T, Prihoda N, Batbold O, Yurchenko L, Tseveendorj A, Arzhanova O, Chunt E, Stepanenko H, Sokolenko N, Makeeva N, Tarakanovskaya M, Borisova V, Reid A, Kalashnikov V, Nyasulu P, Prabowo SA, Jirathitikal V, Bain AI, Stanford C, Stanford J (2019) Phase III, placebo-controlled, randomized, double-blind trial of tableted, therapeutic TB vaccine (V7) containing heat-killed M. vaccae administered daily for one month. J Clin Tuberc Other Mycobact Dis 18:100141
Lei JP, **ong GL, Hu QF, Li Y, Zong PL, Tu SH, Tu RY (2008) Immunotherapeutic efficacy of BCG vaccine in pulmonary tuberculosis and its preventive effect on multidrug-resistant tuberculosis. Zhonghua Yu Fang Yi Xue Za Zhi 42(2):86–89
Prabowo SA, Painter H, Zelmer A, Smith SG, Seifert K, Amat M, Cardona PJ, Fletcher HA (2019) RUTI vaccination enhances inhibition of mycobacterial growth ex vivo and induces a shift of monocyte phenotype in mice. Front Immunol 10:894
Bruffaerts N, Huygen K, Romano M (2014) DNA vaccines against tuberculosis. Expert Opin Biol Ther 14(12):1801–1813. https://doi.org/10.1517/14712598.2014.951630
Liang Y, Bai X, Zhang J, Song J, Yang Y, Yu Q, Li N, Wu X (2016) Ag85A/ESAT-6 chimeric DNA vaccine induces an adverse response in tuberculosis-infected mice. Mol Med Rep 14(2):1146–1152. https://doi.org/10.3892/mmr.2016.5364
Wowk PF, Franco LH, Fonseca DMD, Paula MO, Vianna ÉDSO, Wendling AP, Augusto VM, Elói-Santos SM, Teixeira-Carvalho A, Silva FDC, Vinhas SA, Martins-Filho OA, Palaci M, Silva CL, Bonato VLD (2017) Mycobacterial Hsp65 antigen upregulates the cellular immune response of healthy individuals compared with tuberculosis patients. Hum Vaccin Immunother 13(5):1040–1050
Xu Z, Hu T, Liu Z, Shen X, Liu J, Yin Y, Sun L, Chen X, Jiao X (2016) Expression and immunogenicity of Ag85A protein of Mycobacterium tuberculosis. Wei Sheng wu xue bao = Acta Microbiol Sinica 56(5):804–813
Mir SA, Verma I, Sharma S (2014) Immunotherapeutic potential of recombinant ESAT-6 protein in mouse model of experimental tuberculosis. Immunol Lett 158(1–2):88–94
Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, Kaneda Y, Yoshida S, Nishida Y, Nakatani H, Takao K, Kishigami C, Nishimatsu S, Sekine Y, Inoue Y, Matsumoto M, McMurray DN, De la Cruz EC, Tan EV, Abalos RM, Burgos JA, Saunderson P, Sakatani M (2011) Novel therapeutic vaccine: granulysin and new DNA vaccine against tuberculosis. Hum Vaccin 7:60–67. https://doi.org/10.4161/hv.7.0.14563
Tanghe A, D’Souza S, Rosseels V, Denis O, Ottenhoff TH, Dalemans W, Wheeler C, Huygen K (2001) Improved immunogenicity and protective efficacy of a tuberculosis DNA vaccine encoding Ag85 by protein boosting. Infect Immun 69(5):3041–3047
Derrick SC, Yang AL, Morris SL (2004) A polyvalent DNA vaccine expressing an ESAT6-Ag85B fusion protein protects mice against a primary infection with Mycobacterium tuberculosis and boosts BCG-induced protective immunity. Vaccine 23(6):780–788
Butov DO, Zaitseva SI, Pitenko MM, Stepanenko GL, Butova TS (2015) Morphological changes in experimental tuberculosis resulting from treatment with quercetin and polyvinylpyrrolidone. Int J Mycobacteriol 4(4):296–301
Senderovitz T, Viskum K (1994) Corticosteroids and tuberculosis. Respir Med 88(8):561–565
Schutz C, Davis AG, Sossen B, Lai RP, Ntsekhe M, Harley YX, Wilkinson RJ (2018) Corticosteroids as an adjunct to tuberculosis therapy. Expert Rev Respir Med 12(10):881–891. https://doi.org/10.1080/17476348.2018.1515628
Liu T, Zhang L, Joo D, Sun SC (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023. https://doi.org/10.1038/sigtrans.2017.23
Gräb J, Suárez I, van Gumpel E, Winter S, Schreiber F, Esser A, Hölscher C, Fritsch M, Herb M, Schramm M, Wachsmuth L, Pallasch C, Pasparakis M, Kashkar H, Rybniker J (2019) Corticosteroids inhibit Mycobacterium tuberculosis-induced necrotic host cell death by abrogating mitochondrial membrane permeability transition. Nat Commun 10(1):688. https://doi.org/10.1038/s41467-019-08405-9
Butov D, Feshchenko Y, Kuzhko M, Gumenuik M, Yurko K, Grygorova A, Tkachenko A, Nekrasova N, Tlustova T, Kikinchuk V, Peshenko A, Butova T (2020) Effectiveness of intravenous isoniazid and ethambutol administration in patients with tuberculosis meningoencephalitis and HIV infection. Tuberc Respir Dis (Seoul) 83(1):96–103
Prasad K, Singh MB, Ryan H (2016) Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev 4(4):CD002244
Muefong CN, Sutherland JS (2020) Neutrophils in tuberculosis-associated inflammation and lung pathology. Front Immunol 11:962. https://doi.org/10.3389/fimmu.2020.00962
Critchley JA, Orton LC, Pearson F (2014) Adjunctive steroid therapy for managing pulmonary tuberculosis. Cochrane Database Syst Rev 2014(11):CD011370
Johnson JR, Turk TL, Macdonald FM (1967) Corticosteroids in pulmonary tuberculosis. Am Rev Respir Dis 96:6–73
George IA, Thomas B, Sadhu JS (2018) Systematic review and meta-analysis of adjunctive corticosteroids in the treatment of tuberculous pericarditis. Int J Tuberc Lung Dis 22(5):551–556
Ryan H, Yoo J, Darsini P (2017) Corticosteroids for tuberculous pleurisy. Cochrane Database Syst Rev 3(3):CD001876
Sun F, Li L, Liao X, Yan X, Han R, Lei W, Cao H, Feng M, Cao G (2018) Adjunctive use of prednisolone in the treatment of free-flowing tuberculous pleural effusion: a retrospective cohort study. Respir Med 139:86–90
Donovan J, Phu NH, Mai NTH, Dung LT, Imran D, Burhan E, Ngoc LHB, Bang ND, Giang DC, Ha DTM, Day J, Thao LTP, Thuong NT, Vien NN, Geskus RB, Wolbers M, Hamers RL, van Crevel R, Nursaya M, Maharani K, Hien TT, Baird K, Lan NH, Kestelyn E, Chau NVV, Thwaites GE (2018) Adjunctive dexamethasone for the treatment of HIV-infected adults with tuberculous meningitis (ACT HIV): study protocol for a randomised controlled trial. Wellcome Open Res 3:31
Elliott AM, Luzze H, Quigley MA, Nakiyingi JS, Kyaligonza S, Namujju PB, Ducar C, Ellner JJ, Whitworth JA, Mugerwa R, Johnson JL, Okwera A (2004) A randomized, double-blind, placebo-controlled trial of the use of prednisolone as an adjunct to treatment in HIV-1-associated pleural tuberculosis. J Infect Dis 190(5):869–878
Mayanja-Kizza H, Jones-Lopez E, Okwera A, Wallis RS, Ellner JJ, Mugerwa RD, Whalen CC, Uganda-Case Western Research Collaboration (2005) Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: a phase 2 clinical trial in Uganda. J Infect Dis 191(6):856–865
Ralph AP, Kelly PM, Anstey NM (2008) l-Arginine and vitamin D: novel adjunctive immunotherapies in tuberculosis. Trends Microbiol 16(7):336–344. https://doi.org/10.1016/j.tim.2008.04.003
Brighenti S, Bergman P, Martineau AR (2018) Vitamin D and tuberculosis: where next? J Intern Med. https://doi.org/10.1111/joim.12777.doi:10.1111/joim.12777
Kearns MD, Tangpricha V (2014) The role of vitamin D in tuberculosis. J Clin Transl Endocrinol 1(4):167–169. https://doi.org/10.1016/j.jcte.2014.08.002
Talat N, Perry S, Parsonnet J, Dawood G, Hussain R (2010) Vitamin D deficiency and tuberculosis progression. Emerg Infect Dis 16(5):853–855. https://doi.org/10.3201/eid1605.091693
Chung C, Silwal P, Kim I, Modlin RL, Jo EK (2020) Vitamin D-cathelicidin axis: at the crossroads between protective immunity and pathological inflammation during infection. Immune Netw 20(2):e12. https://doi.org/10.4110/in.2020.20.e12
Junaid K, Rehman A (2019) Impact of vitamin D on infectious disease-tuberculosis—a review. Clin Nutr Exp 25:1–10. https://doi.org/10.1016/j.yclnex.2019.02.003
Torres-Juarez F, Cardenas-Vargas A, Montoya-Rosales A, González-Curiel I, Garcia-Hernandez MH, Enciso-Moreno JA, Hancock RE, Rivas-Santiago B (2015) LL-37 immunomodulatory activity during Mycobacterium tuberculosis infection in macrophages. Infect Immun 83(12):4495–4503. https://doi.org/10.1128/IAI.00936-15
Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC (2013) Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J Biol Chem 288(27):19450–19458. https://doi.org/10.1074/jbc.M113.467670
Adamczak DM (2017) The role of toll-like receptors and vitamin D in cardiovascular diseases—a review. Int J Mol Sci 18(11):2252. https://doi.org/10.3390/ijms18112252
Panwar A, Garg RK, Malhotra HS, Jain A, Singh AK, Prakash S, Kumar N, Garg R, Mahdi AA, Verma R, Sharma PK (2016) 25-Hydroxy vitamin D, vitamin D receptor and toll-like receptor 2 polymorphisms in spinal tuberculosis: a case-control study. Medicine (Baltimore) 95(17):e3418. https://doi.org/10.1097/MD.0000000000003418
Campbell GR, Spector SA (2012) Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy 8(10):1523–1525. https://doi.org/10.4161/auto.21154
Sarkar K, Sil PC (2019) Infectious lung diseases and endogenous oxidative stress. Oxidative Stress in Lung Dis 125–148. https://doi.org/10.1007/978-981-13-8413-4_7
Shastri MD, Shukla SD, Chong WC, Dua K, Peterson GM, Patel RP, Hansbro PM, Eri R, O’Toole RF (2018) Role of oxidative stress in the pathology and management of human tuberculosis. Oxid Med Cell Longev 2018:7695364. https://doi.org/10.1155/2018/7695364
Wang CH, Lin HC, Liu CY, Huang KH, Huang TT, Yu CT, Kuo HP (2001) Upregulation of inducible nitric oxide synthase and cytokine secretion in peripheral blood monocytes from pulmonary tuberculosis patients. Int J Tuberc Lung Dis 5(3):283–291
Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, Kwiatkowski D (1998) 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun 66(11):5314–5321
Butov DO, Kuzhko MM, Kalmykova IM, Kuznetsova IM, Butova TS, Grinishina OO, Maksimenko OA (2014) Changes in nitric oxide synthase and nitrite and nitrate serum levels in patients with or without MDR-TB undergoing the intensive phase of anti-tuberculosis therapy. Int J Mycobacteriol 3(2):139–143
Ralph AP, Waramori G, Pontororing GJ, Kenangalem E, Wiguna A, Tjitra E, Sandjaja LDB, Yeo TW, Chatfield MD, Soemanto RK, Bastian I, Lumb R, Maguire GP, Eisman J, Price RN, Morris PS, Kelly PM, Anstey NM (2013) l-Arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. PLoS ONE 8(8):e70032. https://doi.org/10.1371/journal.pone.0070032
Jolliffe DA, Ganmaa D, Wejse C, Raqib R, Haq MA, Salahuddin N, Daley PK, Ralph AP, Ziegler TR, Martineau AR (2019) Adjunctive vitamin D in tuberculosis treatment: meta-analysis of individual participant data. Eur Respir J 53(3):1802003. https://doi.org/10.1183/13993003.02003-2018
Wu HX, **ong XF, Zhu M, Wei J, Zhuo KQ, Cheng DY (2018) Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: a systematic review and meta-analysis. BMC Pulm Med 18(1):108. https://doi.org/10.1186/s12890-018-0677-6 [Published online 2018 Jun 28]
Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, Maunsell ZJ, Rainbow SJ, Berry JL, Griffiths CJ (2007) A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med 176(2):208–213. https://doi.org/10.1164/rccm.200701-007OC
Nursyam EW, Amin Z, Rumende CM (2006) The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones 38(1):3–5
Zhang J, Chen C, Yang J (2019) Effectiveness of vitamin D supplementation on the outcome of pulmonary tuberculosis treatment in adults: a meta-analysis of randomized controlled trials. Chin Med J (Engl) 132(24):2950–2959. https://doi.org/10.1097/CM9.0000000000000554
Wang J, Feng M, Ying S, Zhou J, Li X (2018) Efficacy and safety of vitamin D supplementation for pulmonary tuberculosis: a systematic review and meta-analysis. Iran J Public Health 47(4):466–472
Ganmaa D, Munkhzul B, Fawzi W, Spiegelman D, Willett WC, Bayasgalan P, Baasansuren E, Buyankhishig B, Oyun-Erdene S, Jolliffe DA, Xenakis T, Bromage S, Bloom BR, Martineau AR (2017) High-dose vitamin D3 during tuberculosis treatment in Mongolia. A randomized controlled trial. Am J Respir Crit Care Med 196(5):628–637. https://doi.org/10.1164/rccm.201705-0936OC
Feng M, Ding Q, Zhong C, Li J, Wang Q, Yuan Z, Dong Y (2016) Adjunctive therapy with V-5 Immunitor (V5) for the treatment of tuberculosis patients: a meta-analysis. Pharmazie 71(9):499–503. https://doi.org/10.1691/ph.2016.6051
Batdelger D, Dandii D, Jirathitikal V, Bourinbaiar AS (2008) Open-label trial of therapeutic immunization with oral V-5 Immunitor (V5) vaccine in patients with chronic hepatitis C. Vaccine 26(22):2733–2737
Butov DA, Pashkov YN, Stepanenko AL, Choporova AI, Butova TS, Batdelger D, Jirathitikal V, Bourinbaiar AS, Zaitzeva SI (2011) Phase IIb randomized trial of adjunct immunotherapy in patients with first-diagnosed tuberculosis, relapsed and multi-drug-resistant (MDR) TB. J Immune Based Ther Vaccines 9:3
Skrahin A, Ahmed RK, Ferrara G, Rane L, Poiret T, Isaikina Y, Skrahina A, Zumla A, Maeurer MJ (2014) Autologous mesenchymal stromal cell infusion as adjunct treatment in patients with multidrug and extensively drug-resistant tuberculosis: an open-label phase 1 safety trial. Lancet Respir Med 2(2):108–122
Hogan BL, Yingling JM (1998) Epithelial/mesenchymal interactions and branching morphogenesis of the lung. Curr Opin Genet Dev 8(4):481–486
Sinclair K, Yerkovich ST, Chambers DC (2013) Mesenchymal stem cells and the lung. Respirology 18(3):397–411
Joshi L, Chelluri LK, Gaddam S (2015) Mesenchymal stromal cell therapy in MDR/XDR tuberculosis: a concise review. Arch Immunol Ther Exp (Warsz) 63(6):427–433
de Vallière S, Abate G, Blazevic A, Heuertz RM, Hoft DF (2005) Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun 73(10):6711–6720
Roy E, Stavropoulos E, Brennan J, Coade S, Grigorieva E, Walker B, Dagg B, Tascon RE, Lowrie DB, Colston MJ, Jolles S (2005) Therapeutic efficacy of high-dose intravenous immunoglobulin in Mycobacterium tuberculosis infection in mice. Infect Immun 73(9):6101–6109
Lopez Y, Yero D, Falero-Diaz G, Olivares N, Sarmiento ME, Sifontes S, Solis RL, Barrios JA, Aguilar D, Hernández-Pando R, Acosta A (2009) Induction of a protective response with an IgA monoclonal antibody against Mycobacterium tuberculosis 16kDa protein in a model of progressive pulmonary infection. Int J Med Microbiol 299(6):447–452
Butova T, Zaitseva S, Butov D, Stepanenko G (2016) Morphological changes in experimental tuberculosis resulting from treatment with quercetin and polyvinylpyrrolidone. Int J Mycobacteriol 5(Suppl 1):S103–S104
Butov D, Zaitseva S, Butova T (2016) Efficacy and safety of quercetin and polyvinylpyrrolidone in treatment of patients with newly diagnosed destructive pulmonary tuberculosis in comparison with standard antimycobacterial therapy. Int J Mycobacteriol. 5(Suppl 1):S110–S111
Batbold U, Butov DO, Kutsyna GA, Damdinpurev N, Grinishina EA, Mijiddorj O, Kovolev ME, Baasanjav K, Butova TS, Sandagdorj M, Batbold O, Tseveendorj A, Chunt E, Zaitzeva SI, Stepanenko HL, Makeeva NI, Mospan IV, Pylypchuk VS, Rowe JL, Nyasulu P, Jirathitikal V, Bain AI, Tarakanovskaya MG, Bourinbaiar AS (2017) Double-blind, placebo-controlled, 1:1 randomized Phase III clinical trial of Immunoxel honey lozenges as an adjunct immunotherapy in 269 patients with pulmonary tuberculosis. Immunotherapy 9(1):13–24
Mezentseva MV, Stakhanov VA, Zakharova MV, Zotova IF, Shapoval IM, Tregubova MI, Russu L (2011) Prospects of immunotherapy in the complex treatment of the infiltrative pulmonary tuberculosis. Biopreparats (Biopharmaceuticals) 2:20–25
Svistunova AS, Pinegin BV, Selitskaia RP (2002) Primenenie immunomoduliatora likopida v kompleksnom lechenii tuberkuleza legkikh [The use of immunomodulator likopid in the combined treatment pulmonary tuberculosis]. Probl Tuberk 3:21–25
Curtis N, Sparrow A, Ghebreyesus TA, Netea MG (2020) Considering BCG vaccination to reduce the impact of COVID-19. Lancet 395(10236):1545–1546
Grange JM, Brunet LR, Rieder HL (2011) Immune protection against tuberculosis—when is immunotherapy preferable to vaccination? Tuberculosis (Edinb) 91(2):179–185
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Butov, D., Myasoedov, V., Tkachenko, A., Butova, T. (2023). Immune Approaches in Tuberculosis Treatment. In: Rezaei, N. (eds) Tuberculosis. Integrated Science, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-031-15955-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-15955-8_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15954-1
Online ISBN: 978-3-031-15955-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)