Summary
As children develop paucibacillary forms of tuberculosis (TB), bacteriological tests usually are negative. This chapter describes aspects of the laboratory tests available for clinical practice. It also discusses perspectives, such as biomarkers, which can be utilized in rapid tests, point of care (POC) tests, performed on samples other than sputum.
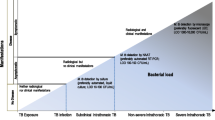
Graphical Abstract

Pediatric tuberculosis diagnosis
Many vague concepts on the pathogenesis of tuberculosis are attributable to an inadequate understanding of the basic facts and principles of the action of the bacteria and the reactions of the host.
Arnold Rich [1]
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Rich A (1946) Patogenia de la tuberculosis. Buenos Aires, Alfa
World Health Organization (2019) Global tuberculosis report 2019. Available https://www.who.int/tb/publications/global_report/en/. Acessed 22 Jun 2020
Sant’Anna CC, Schmidt CM, March MFBP, Pereira SM, Barreto ML (2013) Tuberculosis among adolescents in two Brazilian State capitals. Cad Saúde Pública 29(1):111–116
Carvalho ACC, Cardoso CAA, Martire TM, Migliori GB, Sant’Anna CC (2018) Aspectos epidemiológicos, manifestações clínicas e prevenção da tuberculose pediátrica sob a perspectiva da Estratégia End TB. J Bras Pneumol 44(2):134–144
Gie R (2003) Diagnostic atlas of intrathoracic tuberculosis in children. International Union Against Tuberculosis and Lung Disease, Paris
Ministério da Saúde (2019) Manual de Recomendações para o Controle da Tuberculose no Brasil. Available http://bvsms.saude.gov.br/bvs/publicacoes/manual_recomendacoes_controle_tuberculose_brasil_2_ed.pdf. Acessed 22 Jun 2020
Nonyane BAS, Nicol MP, Andreas NJ, Rimmele S, Schneiderhan-Marra N, Workman LJ, Perkins MD, Joos T, Broger T, Ellner JJ, Alland D, Kampmann B, Dorman SE, Zar HJ (2018) Serologic responses in childhood pulmonary tuberculosis. Pediatr Infect Dis J 37(1):1–9
Graham SM, Cuevas LE, Jean-Philippe P, Browning R, Casenghi RM, Detjen AK, Gnanashanmugam D, Hesseling AC, Kampmann B, Mandalakas A, Marais BJ, Schito M, Spiegel HML, Starke JR, Worrell C, Zar HJ (2015) Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis 61(Suppl 3):S179–S187
World Health Organization (2014) The End TB strategy global strategy and targets for tuberculosis prevention, care and control after 2015. Available https://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1. Acessed 22 Jul 2020
Lobue P, Menzies D (2010) Treatment of latent tuberculosis infection: an update. Respirology 15(4):603–622
World Health Organization (2017) Multidrug-resistant tuberculosis (MDR-TB) 2017 update. Available https://www.who.int/tb/challenges/mdr/MDR-RR_TB_factsheet_2017.pdf?ua=1. Acessed 22 Jul 2020
World Health Organization (2020) WHO consolidated guidelines on tuberculosis module 3: diagnosis rapid diagnostics for tuberculosis detection. Available https://www.who.int/publications/i/item/who-consolidated-guidelines-on-tuberculosis-module-3-diagnosis---rapid-diagnostics-for-tuberculosis-detection. Acessed 22 Jul 2020
Yerlikaya S, Broger T, MacLean E, Yerlikaya S, Broger T, MacLean E, Pai M, Denkinger CM (2017) A tuberculosis biomarker database: the key to novel TB diagnostics. Int J Infect Dis 56:253–257
Haas CT, Roe JK, Pollara G, Mehta M, Noursadeghi M (2016) Diagnostic ‘omics’ for active tuberculosis. BMC Med 14:37
Wessels G, Schaaf HS, Beyers N, Gie RP, Nel E, Donald PR (1999) Haematological abnormalities in children with tuberculosis. J Trop Pediatr 45(5):307–310
Abakay O, Abakay A, Sen HS, Tanrikulu AC (2015) The relationship between inflammatory marker levels and pulmonary tuberculosis severity. Inflammation 38(2):691–696
Peresi E, Silva SMR, Calvi SA, Marcondes-Machado J (2008) Cytokines and acute phase serum proteins as markers of inflammatory regression during the treatment of pulmonary tuberculosis. J Bras de Pneumol 34(11):942–949
Martins C, Gama ACC, Valcarenghi D, Batschauer APB (2014) Markers of acute-phase response in the treatment of pulmonary tuberculosis. J Bras Patol Med Lab 50(6):428–433
Nagu TJ, Spiegelman D, Hertzmark E, Aboud S, Makani J, Matee MI, Fawzi W, Mugusi F (2014) Anemia at the initiation of tuberculosis therapy is associated with delayed sputum conversion among pulmonary tuberculosis patients in Dar-es-Salaam, Tanzania. PLoSOne 18;9(3):e91229
Starke JR, Committee on Infectious Diseases (2014) Interferon-γ release assays for diagnosisoftuberculosisinfectionanddisease in children. Pediatrics 134(6):e1763–e1773
Gonzalez NE, Ferrero F (2015). Diagnóstico da Tuberculose na Infância. In: Tuberculose em crianças e jovens, 1st ed. Editora Atheneu, São Paulo, pp 17–24
Committee on Infectious Diseases, American Academy of Pediatrics (2018) Tuberculosis: 2018–2021 report of the committee on infectious diseases. 31th ed. Elk Grove Village IL pp 829–834
Starke JR (2012) Interferon-gama release assays for the diagnosis of tuberculosis Infection in children. J Pediatrics 161(4):581–582
Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y Hatherill M, Moyo S, Hanekom W, Mahomed H (2011) The utility of interferon-gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr Infect Dis J 30:694–700
World Health Organization (2015) Implementing tuberculosis diagnostic. Policy framework. Available https://apps.who.int/iris/bitstream/handle/10665/162712/9789241508612_eng.pdf;jsessionid=C5ACAB489566CAFC4731CEF90864F9A4?sequence=1. Acessed 01 Aug 2020
Schaaf HS, Reuter H (2009) Practical approaches to ordering diagnostic tests. In: Schaaf HS, Zumla A (eds) Tuberculosis: a comprehensive clinical reference. Saunders, Elsevier, pp 216–226
Martire TM (2009) Diagnóstico laboratorial da tuberculose na infância: métodos convencionais e métodos rápidos. Pulmão RJ Supl 1:20-S27
Cruciani M, Scarparo C, Malena M, Bosco O, Serpelloni G, Mengoli C (2004) Meta-analysis of BACTEC MGIT 960 and BACTEC 460 TB, with or without solid media, for detection of mycobacteria. J Clin Microbiol 42:2321–2325
Nicol MP, Zar HJ (2011) New specimens and laboratory diagnostics for childhood pulmonary TB: progress and prospects. Paediatr Respir Rev 12:16–21
Canetti G, Rist N, Grosset J (1963) Mesure de sensibilité du bacille tuberculeux aux drogues antibacillaire par la methode de proportions: methodologie, criterie de rèsitance, resultats et interpretations. Rev tub pneumol 27:217–272
Moore DA, Mendoza D, Gilman RH, Evans CAW, Delgado MGH, Guerra J, Caviedes L, Vargas D, Ticona E, Ortiz J, Soto G, Serpa J, Tuberculosis Working Group in Peru (2004) Microscopic observation drug susceptibility assay, a rapid, reliable diagnostic test for multidrug-resistant tuberculosis suitable for use in resource-poor settings. J Clin Microbiol 42(10):4432–4437
Ha DT, Lan NT, Wolbers M, Duong TN, Quang ND, Thi Van Thinh T, Thi Hong Ngoc L, Thi Ngoc Anh N, Van Quyet T, Thi Bich Tuyen N, Thi Ha V, Day J, Thi Thanh Hang H, Kiet VS, Thi Nho N, Hoa DV, Dung NH, Huu Lan N, Farrar J, Caws M (2009) Microscopic observation drug susceptibility assay (MODS) for early diagnosis of tuberculosis in children. PLoS ONE 4:e8341
Ministério da Saúde (2011) Manual de Recomendações para o Controle da Tuberculose no Brasil. Available http://bvsms.saude.gov.br/bvs/publicacoes/manual_recomendacoes_controle_tuberculose_brasil_2_ed.pdf. Acessed 22 Jun 2020
Nicol M, Whitelaw A, Stevens W (2013) Using Xpert M. TB/RIF. Curr Respir Med Rev 9(3):187–192
Lange C, Dheda K, Chesov D, Mandalakas AM, Udwadia Z, Horsburgh CR Jr (2019) Management of drug-resistant tuberculosis. The Lancet 394(10202):953–966
Ministério da Saúde (2016) Teste rápido molecular para tuberculose.Nova tecnologia para o diagnóstico da tuberculose. http://www.saude.gov.br/images/pdf/2016/maio/18/folder-TRM-TB-grafica-reduzido.pdf. Acessed 30 Aug 2020
Chakravorty S, Simmons AM, Rowneki M, Parmar H, Yuan Cao Y, Ryan J, Banada PP, Srinidhi Deshpande S, Shubhada Shenai S, Alexander Gall A, Glass , Krieswirth B, Schumacher SG, Nabeta P, Tukvadze N, Rodrigues C, Skrahina A, Tagliani E, Cirillo DM, Davidow A, Denkinger CM, Persing D, Kwiatkowski R, Jones M, Allanda D (2017) The new xpert M. TB/RIF ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-caretesting. mBio 29;8(4):e00812–e00817
World Health Organization (2017) Meeting report of a technical expert consultation: non-inferiority analysis of Xpert M. TB/RIF ultra compared toXpert M. TB/RIF. 11 p. Available https://www.who.int/tb/publications/2017/XpertUltra/en/. Acessed 01 Jul 2020
Sieiro TA, Aurilio RB, Soares ECC, Chiang SS, Sant`Anna CC (2018) The role of the Xpert M. TB/RIF assay among adolescents suspected of pulmonary tuberculosis in Rio de Janeiro, Brazil. Rev Soc Bras Med Trop 51(2):234–236
Atherton RR, Cresswell FV, Ellis J, Kitaka SB, Boulware DR (2019) Xpert M. TB/RIF Ultra for tuberculosis testing in children: a mini-review and commentary. Front Pediatr 7:34
Detjen AK, DiNardo AR, Leyden J, Steingart KR, Menzies D, Schiller I, Dendukuri N, Mandalakas AM (2015) Xpert M. TB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. The Lancet Resp Med 3(6):451–61
Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N (2014) Xpert® M. TB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults (Review). Cochrane Database Of Systematic Reviews. Available https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4470349/pdf/CD009593.pdf. Acessed 10 May 2020
Ioos V, Cordel H, Bonnet M (2018) Alternative sputum collection methods for diagnosis of childhood intrathoracic tuberculosis: a systematic literature review. Arch Dis Child 104(7):629–635
Walters E, Goussard P, Bosch C, Hesseling AC, Gie RP (2013) GeneXpert M. TB/RIF on bronchoalveolar lavage samples in children with suspected complicated intrathoracic tuberculosis: a pilot study. Pediatr Pulmonol 55:1133–1137
Saini I, Mukherjee A, Gautam H, Singla M (2018) Diagnostic yield of Xpert M. TB/RIF in Bronchoalveolar lavage in children with probable pulmonary tuberculosis. Indian Pediatr 55(12):1062–1065
Bholla M, Kapalata N, Masika E, Chande H, Jugheli L, Sasamalo M, Glass TR, Peter Beck H-P, Reither K (2016) Evaluation of Xpert® M. TB/RIF and Ustar Easy NAT™ TB IAD for diagnosis of tuberculous lymphadenitis of children in Tanzania: a prospective descriptive study. BMC Infect Dis 16(1):1–9. Springer Science and Business Media LLC
Aruna J, Ratageri VH, Illalu S, Fattepur SR, Wari PK (2019) The utility of CSF xpert M. TB/RIF in diagnosis of tubercular meningitis in children. Indian J Pediatr 86(12):1089–1093
Lopez AL, Aldaba JG, Morales-Dizon M, Sarol JN, Daag JV, Ama MC, Sylim P, Salonga A, Nielsen-Saines K (2019) Urine Xpert M. TB/RIF for the diagnosis of childhood tuberculosis. Int J Infect Dis 79:44–46
Maynard-Smith L, Larke N, Peters JA, Lawn SD (2014) Diagnostic accuracy of the Xpert M. TB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infect Dis 14(1):1–15
Fantahun M, Kebede A, Yenew B, Gemechu T, Mamuye Y, Tadesse M, Brhane B, Jibriel A, Solomon D, Yaregal Z (2019) Diagnostic accuracy of Xpert M. TB/RIF assay and non-molecular methods for the diagnosis of tuberculosis lymphadenitis. Shankar EM, PLoS ONE 14(9):e0222402
Nicol MP, Worman L, Prins M Bateman L, Ghebrekristos Y, Mbhele S, Denkinger CM, Zar HJ (2018) Accuracy of xpert M. tb/Rif ultra for the diagnosis of pulmonary tuberculosis in children. Pediatr Infect Dis J. 37(10):e261–e263
MacLean E, Kohli M, Weber SF, Suresh A, Schumacher SG, Denkinger CM, Pai M (2020) Advances in molecular diagnosis of TB. J Clin Microbiol. Accepted Manuscript posted on line
Singh S, Singh A, Prajapati S, Kabra SK, Lodha R, Mukherjee A, Singh V, Hesseling AC, Grewal HM (2015) Xpert M. TB/RIF assay can be used on archived gastric aspirate and induced sputum samples for sensitive diagnosis of paediatric tuberculosis. BMC Microbiol 15(1):191
Zar HJ, Workman L, Isaacs W, Munro J, Black F, Eley B, Allen V, Boehme CC, Zemanay W, Nicol MP (2012) Rapid molecular diagnosis of pulmonary tuberculosis in children using nasopharyngeal specimens. Clin Infect Dis 55(8):1088–1095
Zar HJ, Workman LJ, Prins M, Bateman LJ, Mbhele SP, Whitman CB, Denkinger CM, Nicol MP (2019) Tuberculosis diagnosis in children using xpert ultra on different respiratory specimens. Am J Respir Crit Care Med 200(12):1531–1538
Kabir S, Rahman SMM, Ahmed S, Islam MS, Banu RS, Shewade HD, Thekkur P, Anwar S, Banu NA, Nasrin R, Uddin MKM, Choudhury S, Ahmed S, Paul KK, Khatun R, Chisti MJ, Banu S (2020) Xpert ultra assay on stool to diagnose pulmonary tuberculosis in children. Clin Infect Dis. May 18; ciaa583
Doherty TM, Wallis RS, Zumla A Biomarkers of disease activity, cure, and relapse in tuberculosis (2009). Clin Chest Med 30(4):783–96
Biomarkers Definitions Working Group (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69(3):89–95
Wallis RS, Wang C, Doherty TM, Onyebujoh P, Vahedi M, Laang H, Olesen O, Parida S (2010) Zumla A (2010) Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis 10(2):68–69. https://doi.org/10.1016/S1473-3099(10)70003-7
Loots DT (2016) TB or not TB? Improving the understanding and diagnosis of tuberculosis through metabolomics. Biomark Med 10(10):1025–1028
Goodridge A, Cueva C, Lahiff M, Muzanye G, Johnson JL, Nahid P, Riley LW (2012) Anti-phospholipid antibody levels as biomarker for monitoring tuberculosis treatment response. Tuberculosis 92:243–247
Takenami I, de Oliveira CC, Lima FR, Soares J, Machado A Jr, Riley LW, Arruda S (2016) Immunoglobulin G response to mammalian cell entry 1A (Mce1A) protein as biomarker of active tuberculosis. Tuberculosis 100:82–88
Clavijo E, Díaz R, Anguita A, García A, Pinedo A, Smits HL (2003) Comparison of a dipstick assay for detection of Brucella-specific immunoglobulin M antibodies with other tests for serodiagnosis of human brucellosis. Clin Diagn Lab Immunol 10:612–615
Achkar JM, Ziegenbalg A (2012) Antibody responses to mycobacterial antigens in children with tuberculosis: challenges and potential diagnostic value. Clin Vaccine Immunol 19(12):1898–1906
Pan-Hammarström Q, Zhao Y, Hammarström L (2007) Class switch recombination: a comparison between mouse and human. Adv Immunol 93:1–61
Schroeder HW Jr, Mortari F, Shiokawa S, Kirkham PM, Elgavish RA (1995) Bertrand FE 3rd (1995) Developmental regulation of the human antibody repertoire. Ann NY Acad Sci 764:242–260
Schmidt CM, Lovero KL, Carvalho FR, Dos Santos DCM, Barros ACMW, Quintanilha AP, Barbosa AP, Pone MVS, Pone SM, Araujo JM, de Paula Martins C, Macedo SGD, Miceli AL, Vieira ML, Sias SMA, Queiroz A, Coca Velarde LG, Kritski AL, Silva AA, Sant'Anna CC, Riley LW, Araújo Cardoso CA (2020) Serum anti-Mce1A immunoglobulin detection as a tool for differential diagnosis of tuberculosis and latent tuberculosis infection in children and adolescents. Tuberculosis (Edinb) 120:101893
World Health Organization (2011) Tuberculosis serodiagnostic tests policy statement Available https://apps.who.int/iris/bitstream/handle/10665/44652/9789241502054_eng.pdf?sequence=1&isAllowed=y. Acessed 31 Aug 2020
Peter JG, Theron G, van Zyl-Smit R, Haripersad A, Mottay L, Kraus S, Binder A, Meldau R, Hardy A, Dheda K (2012) Diagnostic accuracy of a urine lipoarabinomannan strip-test for TB detection in HIV-infected hospitalised patients. Eur Respir J 40:1211–1220
Nicol MP, Allen V, Workman L, Isaacs W, Munro J, Pienaar S, Black F, Adonis L, Zemanay W, Ghebrekristos Y, Zar HJ (2014) Urine lipoarabinomannan testing for diagnosis of pulmonary tuberculosis in children: a prospective study. Lancet Glob Health May 2(5):e278–84
Wei M, Wu ZY, Lin JH, Li Y, Qian ZX, **e YQ, Su H, Zhou W (2015) Regulation network of serum cytokines induced by tuberculosis-specific antigens reveals biomarkers for tuberculosis diagnosis. Genet Mol Res 14(4):17182–17192
Jenum S, Dhanasekaran S, Ritz C, Macaden R, Doherty TM, Grewal HM, Trials Study Group TB (2016) Added value of IP-10 as a read-out of mycobacterium tuberculosis: specific immunity in young children. Pediatr Infect Dis J 35(12):1336–1338
Sudbury EL, Otero L, Tebruegge M, Messina NL, Seas C, Montes M, Rìos J, Germano S, Gardiner K, Clifford V, Gotuzzo E, Curtis N (2019) Mycobacterium tuberculosis-specific cytokine biomarkers for the diagnosis of childhood TB in a TB-endemic setting. J Clin Tuberc Other Mycobact Dis 16:100102
Togun T, Hoggart CJ, Agbla SC, Gomez MP, Egere U, Sillah AK, Saidy B, Mendy F, Pai M, Kampmann B (2020) A three-marker protein biosignature distinguishes tuberculosis from other respiratory diseases in Gambian children. EBioMedicine 58:102909
Reimand J, Isserlin R, Voisin V, Kucera M, Tannus-Lopes C, Rostamianfar A, Wadi L, Meyer M, Wong J, Xu C, Merico D, Bader GD (2019) Pathway enrichment analysis and visualization of omics data using g:profiler. GSEA, Cytoscape and Enrichment Map Nat Protoc. 14(2):482–517
Flores-Villalva S, Rogríguez-Hernández E, Rubio-Venegas Y, Cantó-Alarcón JG, Milián-Suazo F (2015) What can proteomics tell us about tuberculosis? J Microbiol Biotechnol. 25(8):1181–1194
Oliver SG (2002) Functional genomics: lessons from yeast. Philos Trans R Soc Lond B Biol Sci 357(1417):17–23
National Human Genome Research Institute (NHGRI) (2017) Transcriptome Fact Sheet https://www.genome.gov/13014330/transcriptome-fact-sheet/. Acessed 30 Mar 2017
Anderson ST, Kaforou M, Brent AJ, Wright VJ, Banwell CM, Chagaluka G, Crampin AC, Dockrell HM, French N, Hamilton MS, Hibberd ML, Kern F, Langford PR, Ling L, Mlotha R, Ottenhoff THM, Pienaar S, Pillay V, Scott JAG, Twahir H, Wilkinson RJ, Coin LJ, Heyderman RS, Levin M, Eley B (2014) Diagnosis of childhood tuberculosis and host RNA expression in Africa. N Engl J Med 370(18):1712–1723
Penn-Nicholson A, Hraha T, Thompson EG, Sterling D, Mbandi SK, Wall KM, Fisher M, Suliman S, Shankar S, Hanekom WA, Janjic N, Hatherill M, Kaufmann SHE, Sutherland J, Walzl G, De Groote MA, Ochsner U, Zak DE, Scriba TJ; ACS and GC6–74 cohort study groups (2019) Discovery and validation of a prognostic proteomic signature for tuberculosis progression: a prospective cohort study. PLoS Med 16(4):e1002781
WHO consolidated guidelines on tuberculosis. Module 5: management of tuberculosis in children and adolescents. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Schmidt, C.M., Cardoso, C.A.A., Aurílio, R.B., de Fátima Bazhuni Pombo Sant’ Anna, M., Sant’Anna, C.C. (2023). Pediatric Tuberculosis: Current Evidence for Laboratory Diagnosis. In: Rezaei, N. (eds) Tuberculosis. Integrated Science, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-031-15955-8_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-15955-8_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15954-1
Online ISBN: 978-3-031-15955-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)