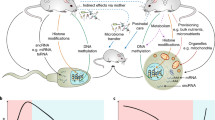
Abstract
Ever since the theory of natural selection was proposed, the study of how characters are inherited across generations has become a principal paramount in biology. These studies have focused on deciphering how phenotypic variation and plasticity across generations contribute to population maintenance and evolution. In this regard, studying how the experience of environmental conditions of a parental population influences offspring phenotypic characteristics through epigenetic processes has gained substantial attention in the past decades. In particular, the mechanisms underpinning this type of transgenerational acclimation include maternal provisioning, microbiome transfer, inheritance of epigenetic markers (e.g., DNA methylation, small RNAs, and histone modifications), and behavioral and cultural processes. These phenomena can result in the programming of the next generation and influence their survival and adaptability to changing environmental conditions. To better understand this topic, in the first part of this chapter I will introduce the reader to the scientific framework on which transgenerational epigenetic programming, and non-genetic inheritance in general, finds its roots. In the second part, I revised the concepts of ‘epigenetics’, ‘transgenerational inheritance’, and, ‘programming’, with the purpose of building a solid ground on which we can base an integrated and deeper discussion in the subsequent sections. The third part of this chapter is focused on discussing the connection between these three concepts, as well as to delve into the tight, but complex, link between ‘transgenerational epigenetic programming’ and developmental biology. After revieing the concept and providing examples of its complexity, I discuss the potential evolutionary implications of transgenerational epigenetic programming in the fourth part of the chapter. Finally, I posit a list of topics and approaches that warrant further research in this scientific field and provide future directions that will help to elucidate knowledge gaps.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Krogh’s principle for a new era (2003) Nat Genet 34(4):345–346
Aiken CE, Ozanne SE (2014) Transgenerational developmental programming. Hum Reprod Update 20(1):63–75
Aiken CE, Tarry-Adkins JL, Ozanne SE (2015) Transgenerational developmental programming of ovarian reserve. Sci Rep 5:16175
Alfaradhi MZ, Fernandez-Twinn DS, Martin-Gronert MS, Musial B, Fowden A, Ozanne SE (2014) Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am J Phys Regul Integr Comp Phys 307:R26–R34
Andreas E, Reid M, Zhang W, Moley KH (2019) The effect of maternal high-fat/high-sugar diet on offspring oocytes and early embryo development. Mol Hum Reprod 25:717–728
Anway MD, Rekow SS, Skinner MK (2008) Transgenerational epigenetic programming of the embryonic testis transcriptome. Genomics 91:30–40
Ardura A, Zaiko A, Morán P, Planes S, Garcia-Vazquez E (2017) Epigenetic signatures of invasive status in populations of marine invertebrates. Sci Rep 7:42193
Ausió J, González-Romero R, Woodcock CL (2014) Comparative structure of vertebrate sperm chromatin. J Struct Biol 188:142–155
Bautista NM, Crespel A (2021) Within-and trans-generational environmental adaptation to climate change: perspectives and new challenges. Front Physiol 8:1–12
Bautista NM, Crespel A, Crossley J, Padilla P, Burggren W (2020) Parental transgenerational epigenetic inheritance related to dietary crude oil exposure in Danio rerio. J Exp Biol jeb.222224
Bautista NM, do Amaral-Silva L, Dzialowski E, Burggren WW (2021) Dietary exposure to low levels of crude oil affects physiological and morphological phenotype in adults and their eggs and hatchlings of the king quail (Coturnix chinensis). Front Physiol 12:477
Beddall BG (1968) Wallace, Darwin, and the theory of natural selection: a study in the development of ideas and attitudes. J Hist Biol 1:261–323
Bell MA, Aguirre W (2013) Contemporary evolution, allelic recycling, and adaptive radiation of the threespine stickleback. Evol Ecol Res 15:377–411
Bergman Y, Cedar H (2013) DNA methylation dynamics in health and disease. Nat Struct Mol Biol 20:274–281
Bernatchez L (2016) On the maintenance of genetic variation and adaptation to environmental change: considerations from population genomics in fishes. J Fish Biol 89:2519–2556
Bian Y, Alberio R, Allegrucci C, Campbell KH, Johnson AD (2009) Epigenetic marks in somatic chromatin are remodelled to resemble pluripotent nuclei by amphibian oocyte extracts. Epigenetics 4:194–202
Biswas S, Rao CM (2018) Epigenetic tools (the writers, the readers and the erasers) and their implications in cancer therapy. Eur J Pharmacol 837:8–24
Bonduriansky R, Day T (2018) Extended heredity: a new understanding of inheritance and evolution, p 281
Burggren W (2016) Epigenetic inheritance and its role in evolutionary biology: re-evaluation and new perspectives. Biology 5:1–22
Burggren WW (2017) Epigenetics in insects: mechanisms, phenotypes and ecological and evolutionary implications. Elsevier, pp 1–30
Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ (1998) Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci 95:5335–5340
Cantone I, Fisher AG (2013) Epigenetic programming and reprogramming during development. Nat Struct Mol Biol 20:282–289
Carroll SP, Hendry AP, Reznick DN, Fox CW (2007) Evolution on ecological time-scales. Funct Ecol 21:387–393
Cavalieri V, Spinelli G (2017) Environmental epigenetics in zebrafish. Epigenetics Chromatin 10:46
Cavieres G, Rezende EL, Clavijo-Baquet S, Alruiz JM, Rivera-Rebella C, Boher F, Bozinovic F (2020) Rapid within- and transgenerational changes in thermal tolerance and fitness in variable thermal landscapes. Ecol Evol 10:8105–8113
Charlesworth D, Barton NH, Charlesworth B (2017) The sources of adaptive variation. Proc R Soc B Biol Sci 284:20162864
Chavatte-Palmer P, Velazquez MA, Jammes H, Duranthon V (2018) Review: epigenetics, developmental programming and nutrition in herbivores. Animal 12:s363–s371
Chen M, Zhang L (2011) Epigenetic mechanisms in developmental programming of adult disease. Drug Discov Today 16:1007–1018
Chittka A, Wurm Y, Chittka L (2012) Epigenetics: the making of ant castes. Curr Biol 22:R835–R838
Cummins J (1998) Mitochondrial DNA in mammalian reproduction. Rev Reprod 3:172–182
Darr J (2020) Future perspectives in epigenetic inheritance. Springer International Publishing:231–259
Day T, Bonduriansky R (2011) A unified approach to the evolutionary consequences of genetic and nongenetic inheritance. Am Nat 178:E18–E36
De Kloet ER, Vreugdenhil E, Oitzl MS, JoëLs M (1998) Brain corticosteroid receptor balance in health and disease*. Endocr Rev 19:269–301
Dean W, Santos F, Reik W (2003) Epigenetic reprogramming in early mammalian development and following somatic nuclear transfer. Semin Cell Dev Biol 14:93–100
Díaz-Freije E, Gestal C, Castellanos-Martínez S, Morán P (2014) The role of DNA methylation on Octopus vulgaris development and their perspectives. Front Physiol 5:62
Dickins TE, Dickins BJA (2018) The extent of the modern synthesis: the foundational framework for evolutionary biology. Springer International Publishing, pp 155–176
Dobzhansky T (1982) Genetics and the origin of species. Columbia university press
Dolinoy DC, Huang D, Jirtle RL (2007) Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci 104:13056–13061
Donelson JM, Sunday JM, Figueira WF, Gaitán-Espitia JD, Hobday AJ, Johnson CR, Leis JM, Ling SD, Marshall D, Pandolfi JM, Pecl G, Rodgers GG, Booth DJ, Munday PL (2019) Understanding interactions between plasticity, adaptation and range shifts in response to marine environmental change. Philosophical Transactions of the Royal Society B: Biological Sciences 374:1–14
Duffié R, Bourc'his D (2013) Chapter nine- parental epigenetic asymmetry in mammals. In: Heard E (ed) Current topics in developmental biology. Academic Press, pp 293–328
Egger G, Liang G, Aparicio A, Jones PA (2004) Epigenetics in human disease and prospects for epigenetic therapy. Nature 429:457–463
Elango N, Hunt BG, Goodisman MAD, Yi SV (2009) DNA methylation is widespread and associated with differential gene expression in castes of the honeybee, Apis mellifera. Proc Natl Acad Sci 106:11206–11211
Falconer DS, Mackay TFC (1981) Introduction to quantitative genetics, 2nd edn. Longman Group, New York
Feil R, Fraga MF (2012) Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet 13:97–109
Fetterman JL, Pompilius M, Westbrook DG, Uyeminami D, Brown J, Pinkerton KE, Ballinger SW (2013) Developmental exposure to second-hand smoke increases adult Atherogenesis and alters mitochondrial DNA copy number and deletions in apoE−/− mice. PLoS One 8:e66835
Fleming AS, Fau O'DD, Kraemer GW (1999) Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosci Biobehav Rev 23:673–685
Flores K, Wolschin F, Corneveaux JJ, Allen AN, Huentelman MJ, Amdam GV (2012) Genome-wide association between DNA methylation and alternative splicing in an invertebrate. BMC Genomics 13:480
Forsman A (2015) Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity 115:276–284
Francis D, Diorio J, Liu D, Meaney MJ (1999) Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 286:1155–1158
Frésard L, Morisson M, Brun J-M, Collin A, Pain B, Minvielle F, Pitel F (2013) Epigenetics and phenotypic variability: some interesting insights from birds. Genet Sel Evol 45:16
Futuyma DJ (2015) Can modern evolutionary theory explain macroevolution? Macroevolution Springer:29–85
Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21:394–407
Ghasemi S (2020) Cancer's epigenetic drugs: where are they in the cancer medicines? Pharmacogenomics J 20:367–379
Gilbert SF, Opitz JM, Raff RA (1996) Resynthesizing evolutionary and developmental biology. Dev Biol 173:357–372
Ginder GD, Williams DC (2018) Readers of DNA methylation, the MBD family as potential therapeutic targets. Pharmacol Ther 184:98–111
Gould SJ (2002) The structure of evolutionary theory. Harvard University Press
Guerrero-Bosagna C, Morisson M, Liaubet L, Rodenburg TB, de Haas EN, Košťál Ľ, Pitel F (2018) Transgenerational epigenetic inheritance in birds. Environmental. Epigenetics 4
Guo F, Li X, Liang D, Li T, Zhu P, Guo H, Wu X, Wen L, Gu TP, Hu B, Walsh CP (2014) Active and passive demethylation of male and female pronuclear DNA in the mammalian zygote. Cell Stem Cell 15(4):447–459
Gyllenhammer LE, Entringer S, Buss C, Wadhwa PD (2020) Developmental programming of mitochondrial biology: a conceptual framework and review. Proc R Soc B Biol Sci 287:20192713
Hairston NG, Ellner SP, Geber MA, Yoshida T, Fox JA (2005) Rapid evolution and the convergence of ecological and evolutionary time. Ecol Lett 8:1114–1127
Hammond SA, Nelson CJ, Helbing CC (2016) Environmental influences on the epigenomes of herpetofauna and fish. Biochem Cell Biol 94:95–100
Hanafi MY, Abdelkhalek TM, Saad MI, Saleh MM, Haiba MM, Kamel MA (2016) Diabetes-induced perturbations are subject to intergenerational transmission through maternal line. J Physiol Biochem 72:315–326
Hanif EAM, Shah SA (2018) Overview on epigenetic re-programming: a potential therapeutic intervention in triple negative breast cancers. Asian Pac J Cancer Prev 19:3341–3351
Hitchler M, Domann F (2007) An epigenetic perspective on the free radical theory of development. Free Radic Biol Med 43:1023–1036
Hu X, Chen Y, Zhao ZJ (2015) Structure, regulation, and function of TET family proteins. Elsevier, pp 379–395
Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Álvarez Pérez HJ, Garland T (2009) Why tropical forest lizards are vulnerable to climate warming. Proc R Soc B Biol Sci 276:1939–1948
Huxley J (1942) The modern synthesis. In: Evolution. The modern synthesis. Evolution
Inoue A, Jiang L, Lu F, Suzuki T, Zhang Y (2017) Maternal H3K27me3 controls DNA methylation-independent imprinting. Nature 547:419–424
Ishihara T, Hickford D, Shaw G, Pask AJ, Renfree MB (2019) DNA methylation dynamics in the germline of the marsupial tammar wallaby, Macropus eugenii. DNA Res 26:85–94
Jablonka E, Lamb MJ (2014) Evolution in four dimensions, revised edition: genetic, epigenetic, behavioral, and symbolic variation in the history of life. MIT press
Jablonka E, Lamb MJ (2015) The inheritance of acquired epigenetic variations. Int J Epidemiol 44:1094–1103
Jablonka E, Lamb MJ (2020) Inheritance systems and the extended synthesis. Cambridge University Press
Jablonka E, Raz G (2009) Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. 84:131–176
Jiang L, Zhang J, Wang J-J, Wang L, Zhang L, Li G, Yang X, Ma X, Sun X, Cai J, Zhang J, Huang X, Yu M, Wang X, Liu F, Wu C-I, He C, Zhang B, Ci W, Liu J (2013) Sperm, but not oocyte, DNA Methylome is inherited by zebrafish early embryos. Cell 153:773–784
Jiang Y-H, Bressler J, Beaudet AL (2004) Epigenetics and human disease. Annu Rev Genomics Hum Genet 5:479–510
Jurkowska RZ, Jeltsch A (2016) Enzymology of mammalian DNA Methyltransferases. Springer International Publishing, pp 87–122
Kass SU, Pruss D, Wolffe AP (1997) How does DNA methylation repress transcription? Trends Genet 13:444–449
Klironomos FD, Berg J, Collins S (2013) How epigenetic mutations can affect genetic evolution: model and mechanism. BioEssays 35:571–578
Klosin A, Lehner B (2016) Mechanisms, timescales and principles of trans-generational epigenetic inheritance in animals. Curr Opin Genet Dev 36:41–49
Krebs HA (1975) The august Krogh principle: “for many problems there is an animal on which it can be most conveniently studied”. J Exp Zool 194:221–226
Lacal I, Ventura R (2018) Epigenetic inheritance: concepts, mechanisms and perspectives. Front Mol Neurosci 11:1–22
Laland K, Matthews B, Feldman MW (2016) An introduction to niche construction theory. Evol Ecol 30:191–202
Laland K, Uller T, Feldman M, Sterelny K, Müller GB, Moczek A, Jablonka E, Odling-Smee J, Wray GA, Hoekstra HE, Futuyma DJ, Lenski RE, Mackay TFC, Schluter D, Strassmann JE (2014) Does evolutionary theory need a rethink? Nature 514:161–164
Laland, K.N., Uller, T., Feldman, M.W., Sterelny, K., Mu, G.B., Uller, T., Moczek, A., 2015. The extended evolutionary synthesis: its structure, assumptions and predictions
Langley-Evans SC (2006) Developmental programming of health and disease. Proc Nutr Soc 65:97–105
Lee H, Hore TA, Reik W (2014) Reprogramming the Methylome: erasing memory and creating diversity. Cell Stem Cell 14:710–719
Lewis A, Austin E, Knapp R, Vaiano T, Galbally M (2015) Perinatal maternal mental health, fetal programming and child development. Healthcare 3:1212–1227
Li E, Beard C, Jaenisch R (1993) Role for DNA methylation in genomic imprinting. Nature 366:362–365
Li E, Zhang Y (2014) DNA methylation in mammals. Cold Spring Harb Perspect Biol 6:a019133–a019133
Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky Paul M, Meaney Michael J (1997) Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277:1659–1662
Liu H, Wang X, Wang H-D, Wu J, Ren J, Meng L, Wu Q, Dong H, Wu J, Kao T-Y, Ge Q, Wu Z-X, Yuh C-H, Shan G (2012) Escherichia coli noncoding RNAs can affect gene expression and physiology of Caenorhabditis elegans. Nat Commun 3:1073
Manhard CV, Joyce JE, Gharrett AJ (2017) Evolution of phenology in a salmonid population: a potential adaptive response to climate change. Can J Fish Aquat Sci 74:1519–1527
Mayr E (1982) The growth of biological thought: diversity, evolution, and inheritance. Harvard University Press
Mayr E (1991) One long argument: Charles Darwin and the genesis of modern evolutionary thought. Harvard University Press
Mayr E (1993) What was the evolutionary synthesis? Trends Ecol Evol 8:31–34
Meaney MJ (2001) Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 24:1161–1192
Meaney MJ, Szyf M (2005) Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci 7:103–123
Migicovsky Z, Kovalchuk I (2011) Epigenetic memory in mammals. Front Genet 2:28
Minocherhomji S, Tollefsbol TO, Singh KK (2012) Mitochondrial regulation of epigenetics and its role in human diseases. Epigenetics 7:326–334
Moczek AP, Sultan S, Foster S, Ledón-Rettig C, Dworkin I, Nijhout HF, Abouheif E, Pfennig DW (2011) The role of developmental plasticity in evolutionary innovation. Proc R Soc B Biol Sci 278:2705–2713
Moghadam H, Mørkøre T, Robinson N (2015) Epigenetics–potential for programming fish for aquaculture? J Marine Sci Eng 3:175–192
Moore DS (2015) The develo** genome: an introduction to behavioral epigenetics. Oxford University Press
Morgan R, Finnøen MH, Jensen H, Pélabon C, Jutfelt F (2020) Low potential for evolutionary rescue from climate change in a tropical fish. Proc Natl Acad Sci 117:33365–33372
Nicholson TB, Veland N, Chen T (2015) Writers, readers, and erasers of epigenetic Marks. Elsevier, pp 31–66
Nilsson E, Skinner MK (2014) Chapter 2–definition of epigenetic transgenerational inheritance and biological impacts. In: Tollefsbol T (ed) Transgenerational epigenetics. Academic Press, Oxford, pp 11–16
Nilsson EE, Maamar MB, Skinner MK (2020) Environmentally induced epigenetic transgenerational inheritance and the Weismann barrier: the Dawn of neo-Lamarckian theory. J Develop Biol 8(4):28. https://doi.org/10.3390/jdb8040028
Noble D (2015) Conrad Waddington and the origin of epigenetics. J Exp Biol 218:816 LP -818
Noble D, Jablonka E, Joyner M, Müller GB, Omholt S, Roux E, Badyaev AV, Baverstock K, Rönkkö M, Jaeger J (2014) The integration of evolutionary biology with physiological science. J Physiol 592:2237–2438
Odling-Smee FJ, Laland KN, Feldman MW (1996) Niche construction. Am Nat 147:641–648
Ortega-Recalde O, Day RC, Gemmell NJ, Hore TA (2019) Zebrafish preserve global germline DNA methylation while sex-linked rDNA is amplified and demethylated during feminisation. Nature. Communications 10
Peat JR, Dean W, Clark SJ, Krueger F, Smallwood SA, Ficz G, Kim JK, Marioni JC, Hore TA, Reik W (2014) Genome-wide bisulfite sequencing in zygotes identifies demethylation targets and maps the contribution of TET3 oxidation. Cell Rep 9:1990–2000
Peterside IE, Selak MA, Simmons RA (2003) Impaired oxidative phosphorylation in hepatic mitochondria in growth-retarded rats. Am J Physiol-Endocrinol Metabolism 285:E1258–E1266
Pigliucci, M., Müller, G.B., 2010. Evolution–the extended synthesis
Potok ME, Nix DA, Parnell TJ, Cairns BR (2013) Reprogramming the maternal zebrafish genome after fertilization to match the paternal methylation pattern. Cell 153:759–772
Provine WB (2020) The origins of theoretical population genetics. University of Chicago Press
Qureshi IA, Mehler MF (2018) Epigenetic mechanisms underlying nervous system diseases. Elsevier:43–58
Reznick DN, Losos J, Travis J (2019) From low to high gear: there has been a paradigm shift in our understanding of evolution. Ecol Lett 22:233–244
Richards EJ (2006) Inherited epigenetic variation — revisiting soft inheritance. Nat Rev Genet 7:395
Ruhr I, Bierstedt J, Rhen T, Das D, Singh SK, Miller S, Crossley DA, Galli GLJ (2021) Developmental programming of DNA methylation and gene expression patterns is associated with extreme cardiovascular tolerance to anoxia in the common snap** turtle. Epigenetics Chromatin 14
Ryu T, Veilleux HD, Donelson JM, Munday PL, Ravasi T (2018) The epigenetic landscape of transgenerational acclimation to ocean warming. Nat Clim Chang 8:504–509
Saben JL, Boudoures AL, Asghar Z, Thompson A, Drury A, Zhang W, Chi M, Cusumano A, Scheaffer S, Moley KH (2016) Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep 16:1–8
Sarda S, Zeng J, Hunt BG, Yi SV (2012) The evolution of invertebrate gene body methylation. Mol Biol Evol 29:1907–1916
Sarma RR, Edwards RJ, Crino OL, Eyck HJF, Waters PD, Crossland MR, Shine R, Rollins LA (2020) Do epigenetic changes drive corticosterone responses to alarm cues in larvae of an invasive amphibian? Integr Comp Biol 60:1481–1494
Seidel GE (2002) Chapter 10 - genetic and phenotypic similarity among members of mammalian clonal sets. In: Cibelli J, Lanza RP, Campbell KHS, West MD (eds) Principles of cloning. Academic Press, San Diego, pp 215–225
Seisenberger S, Peat JR, Hore TA, Santos F, Dean W, Reik W (2013) Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philosophical Transactions of the Royal Society B: Biological Sciences 368:20110330
Sharma U, Conine Colin C, Shea Jeremy M, Boskovic A, Derr Alan G, Bing **n Y, Belleannee C, Kucukural A, Serra Ryan W, Sun F, Song L, Carone Benjamin R, Ricci Emiliano P, Li **n Z, Fauquier L, Moore Melissa J, Sullivan R, Mello Craig C, Garber M, Rando Oliver J (2016) Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351:391–396
Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D (2008) Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One 3:e3745
Skvortsova K, Iovino N, Bogdanović O (2018) Functions and mechanisms of epigenetic inheritance in animals. Nat Rev Mol Cell Biol 19:774–790
Smith ZD, Chan MM, Humm KC, Karnik R, Mekhoubad S, Regev A, Eggan K, Meissner A (2014) DNA methylation dynamics of the human preimplantation embryo. Nature 511:611–615
Smith ZD, Chan MM, Mikkelsen TS, Gu H, Gnirke A, Regev A, Meissner A (2012) A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature 484:339–344
Søreide K (2017) Cancer epigenetics. Elsevier, pp 519–534
Stearns T (2001) Centrosome Duplication. Cell 105:417–420
Sultan SE (2015) Organism and environment: ecological development, niche construction, and adaption. Oxford University Press, USA
Suzuki MM, Bird A (2008) DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet 9:465–476
Szyf M (2015) Nongenetic inheritance and transgenerational epigenetics, pp 1–11
Tate PH, Bird AP (1993) Effects of DNA methylation on DNA-binding proteins and gene expression. Curr Opin Genet Dev 3:226–231
Tollefsbol T (2017a) Handbook of epigenetics: the new molecular and medical genetics. Academic Press
Tollefsbol T (2019) Translational epigenetics series. In: Tollefsbol TO (ed) Transgenerational epigenetics, 2nd edn. Academic Press
Tollefsbol TO (2014) Transgenerational epigenetics. Transgenerational Epigenetics Elsevier:1–8
Tollefsbol TO (2017b) An overview of epigenetics. Handbook of Epigenetics:1–6
Torres IO, Fujimori DG (2015) Functional coupling between writers, erasers and readers of histone and DNA methylation. Curr Opin Struct Biol 35:68–75
Uller T, English S, Pen I (2015) When is incomplete epigenetic resetting in germ cells favoured by natural selection? Proc R Soc B Biol Sci 282:1–8
Uller T, Laland KN (2019) Evolutionary causation: biological and philosophical reflections. Mit Press
Vaiserman A (2014) Developmental epigenetic programming of caste-specific differences in social insects: an impact on longevity. Curr Aging Sci 7:176–186
Van Cauwenbergh O, Di Serafino A, Tytgat J, Soubry A (2020) Transgenerational epigenetic effects from male exposure to endocrine-disrupting compounds: a systematic review on research in mammals. Clinical. Epigenetics 12
Veland N, Chen T (2017) Mechanisms of DNA methylation and demethylation during mammalian development. Handbook of Epigenetics Elsevier:11–24
Vickers M, Sloboda D (2012) Strategies for reversing the effects of metabolic disorders induced as a consequence of developmental programming. Front Physiol 3:242
Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M (2005) Neonatal leptin treatment reverses developmental programming. Endocrinology 146:4211–4216
Vinci MC, Polvani G, Pesce M (2013) Epigenetic programming and risk: the birthplace of cardiovascular disease? Stem Cell Rev Rep 9:241–253
Vriens A, Nawrot TS, Baeyens W, Den Hond E, Bruckers L, Covaci A, Croes K, De Craemer S, Govarts E, Lambrechts N, Loots I, Nelen V, Peusens M, De Henauw S, Schoeters G, Plusquin M (2017) Neonatal exposure to environmental pollutants and placental mitochondrial DNA content: A multi-pollutant approach. Environ Int 106:60–68
Waddington CH (1942) The Epigenotype. In: Endeavour, vol 1, pp 18–20
Wallace DC (2016) Mitochondrial DNA in evolution and disease. Nature 535:498–500
Wang L, Zhang J, Duan J, Gao X, Zhu W, Lu X, Yang L, Zhang J, Li G, Ci W, Li W, Zhou Q, Aluru N, Tang F, He C, Huang X, Liu J (2014) Programming and inheritance of parental DNA Methylomes in mammals. Cell 157:979–991
Wang X, Bhandari RK (2019) DNA methylation dynamics during epigenetic reprogramming of medaka embryo. Epigenetics 14:611–622
Wang X, Bhandari RK (2020) DNA methylation reprogramming in medaka fish, a promising animal model for environmental epigenetics research. Environmental. Epigenetics 6
Weaver ICG (2005) Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci 25:11045–11054
Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ (2004a) Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854
Weaver ICG, Diorio J, Seckl JR, Szyf M, Meaney MJ (2004b) Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann N Y Acad Sci 1024:182–212
Weiner SA, Toth AL (2012) Epigenetics in social insects: a new direction for understanding the evolution of castes. Genetics Research International 2012:1–11
Weismann A (1893) The germ-plasm: a theory of heredity. Scribner's
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press
Wild G, Traulsen A (2007) The different limits of weak selection and the evolutionary dynamics of finite populations. J Theor Biol 247:382–390
Wu X, Zhang Y (2017) TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet 18:517–534
Yin J, Zhou M, Lin Z, Li QQ, Zhang YY (2019) Transgenerational effects benefit offspring across diverse environments: a meta-analysis in plants and animals. Ecol Lett 22:1976–1986
Zander-Fox DL, Fullston T, McPherson NO, Sandeman L, Kang WX, Good SB, Spillane M, Lane M (2015) Reduction of mitochondrial function by FCCP during mouse cleavage stage embryo culture reduces birth weight and impairs the metabolic health of offspring 1. Biol Reprod 92:124–124
Zapata-Martín Del Campo C, Martínez-Rosas M, Guarner-Lans V (2018) Epigenetic programming of synthesis, release, and/or receptor expression of common mediators participating in the risk/resilience for comorbid stress-related disorders and coronary artery disease. Int J Mol Sci 19:1224
Zhu Z, Cao F, Li X (2019) Epigenetic programming and fetal metabolic programming. Front Endocrinol 10
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bautista, N.M. (2022). Transgenerational Epigenetic Programming. In: Vaschetto, L.M. (eds) Epigenetics, Development, Ecology and Evolution. Springer, Cham. https://doi.org/10.1007/978-3-031-13771-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-13771-6_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-13770-9
Online ISBN: 978-3-031-13771-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)