Abstract
As a result of increasing globalization, hundreds of forest insect species have been accidentally transported among continents. The most common invasion pathways by which non-native forest insects are transported globally are wood packaging material and live plants. While most non-native forest insect species have no noticeable impacts in their non-native ranges, several species have had serious or even catastrophic effects on tree health, forest resources and ecosystem functions. Approaches to preventing and managing forest insect invasions correspond to the four phases of biological invasions: arrival, establishment, spread and widespread established populations. Biosecurity agencies manage arrival through commodity entry prohibitions, port inspections and mandatory phytosanitary procedures designed to reduce transport of non-native species. Biosecurity measures to prevent establishment focus on surveillance (e.g. traps) to detect new populations that sometimes can be eradicated if populations are discovered early and their occurrence is limited. In very few cases, spread of invasions may be slowed or stopped using containment or barrier zone management. Finally, once populations are established and widespread, methods such as biological control, host resistance breeding, silviculture and pesticides may be needed to mitigate damage caused by non-native pests. As the world becomes increasing interconnected, more insect species are likely to become established in new regions, further increasing the problems associated with non-native forest pests.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
23.1 Introduction
The problem of biological invasions is largely an inadvertent result of globalization. Global trade and human travel have resulted in the accidental movement of organisms across geographic barriers such as oceans and major mountain ranges that previously compartmentalized the world’s flora and fauna through millions of years of evolution. Most non-native organisms established outside their range are inconsequential, with little noticeable impact on invaded ecosystems. However, a fraction of non-native species become extremely abundant and/or greatly alter ecosystem processes and properties (Lovett et al. 2006, 2016). Ever-increasing rates of international trade and travel are likely to provide further opportunities for transport of non-native organisms into new regions.
Given that insects are the most diverse group of organisms in the world, it comes as no surprise that they comprise a large portion of all invading species worldwide (Seebens et al. 2017). Insects exhibit remarkable variation in life histories, and many species require plants for feeding, habitat or both. Among non-native insects that feed on forest trees, there are four major groups of that are particularly damaging: insects that bore through the outer bark of trees to feed on phloem (inner bark) and/or wood, defoliating insects that feed on foliage or within shoots, sap-feeding insects, and seed-eaters. These types of insects are common among non-native forest insects in all regions of the world, with many affecting native forests, tree plantations and urban forests.
Fortunately, most species of non-native forest insects have little impact on trees in their new habitat (Aukema et al. 2010). A fraction of these insects, however, affect tree appearance, growth or vigor, and a small number of species have had catastrophic impacts on invaded forests (Table 23.1). In some cases, such as the invasion of the emerald ash borer, Agrilus planipennis (Buprestidea), in North America (Herms and McCullough 2014), invasion can result in local extirpation of their hosts. Several invasive forest species have greatly altered silvicultural practices. For example, damage caused by the green spruce aphid, Elatiobium abietinum (Aphididae), was so severe in Iceland that planting of spruce was largely abandoned in southern regions (Halldórsson et al. 2003).
Less destructive insect species don’t necessarily kill their host trees and their impacts may be more difficult to quantify. Defoliators, for example, may reduce growth rates, affect form of young trees, and increase vulnerability of severely affected trees to other, secondary pests. A few species facilitate infection by tree pathogens, which can result in considerable damage. For example, the beech scale, Cryptococcus fagisuga (Eriococcidae), which was accidentally introduced to North America and Europe, creates punctures in the outer bark where the tiny beech scales feed. These punctures, allow entry of pathogenic fungi, Nectria spp., which cause beech bark disease and ultimately tree death (Houston 1994). Feeding by the European elm bark beetle, Scolytus multistriatus, (Curculionidae) rarely damages trees but the beetles vector Dutch elm disease, which is typically fatal to American elms. Numerous invasive forest insects, either directly or indirectly, alter ecosystem processes such as nutrient cycling or competitive interactions among plant species (Lovett et al. 2016).
Here we provide a general overview of the causes, ecology and impacts of forest insect invasions, including strategies for managing invasions. We limit our coverage to plant-feeding species, though other feeding guilds (e.g. predators, pollinators) can also have ecological impacts. Other reviews covering forest insect invasions with different areas of focus may be found elsewhere (Niemelä and Mattson 1996; Aukema et al. 2010, 2011; Brockerhoff and Liebhold 2017). Insect invasions are part of a larger problem of biological invasions in forests, and other reviews cover that subject (e.g. Liebhold et al. 1995, 2017a; NRC 2002; Ghelardini et al. 2017; Seebens et al. 2017). Our treatment of this subject is structured using the three universal phases of invasions: arrival, establishment and spread of invading populations.
23.2 Arrival
The problem of biological invasions is largely caused by people moving organisms from their native range into new regions. Mechanisms by which organisms are inadvertently moved around the world are varied and referred to as “invasion pathways”. There are many different invasion pathways responsible for insect introductions (McCullough et al. 2006; Meurisse et al. 2019). Forest insects, however, are most often introduced through one of four invasion pathways: international movement of (i) wood, (ii) plants and plant parts, (iii) hitchhiking (i.e. movement on inanimate or non-host objects) and (iv) intentional introductions (including biological control agents) (Table 23.2). Relatively few forest insect species are thought to have dispersed naturally (e.g. by flight or on wind or water) to new world regions.
An analysis of 62 species of invasive forest insect pests (excluding seed-feeding insects) established in the USA indicated that historically, imports of live plants was responsible for more invasions than any other pathway (Liebhold et al. 2012). Plants imported for propagation are a particularly dominant invasion pathway for sap-feeding and foliage-feeding insects. Live plants represent the “perfect” pathway for many herbivorous insects since most can live and feed on their host plant throughout their journey and upon arrival, the insects already have a suitable host plant that is likely to be nurtured and tended. The importance of live plant imports as a pathway for insect invasions has been confirmed in many world regions (Kiritani and Yamamura 2003; Roques et al. 2009). There have been substantial advances in develo** biosecurity measures designed to limit accidental insect invasions with commercially imported plants (Liebhold and Griffin 2016). These policies vary among world regions, however, and regulation of plant imports in some regions is weak (Eschen et al. 2015).
Not surprisingly, most non-native insects that feed beneath bark on phloem or wood are introduced with imported logs or wood. Some invasions of bark- and wood-borers, such as the introduction of the European elm bark beetle to North America, are attributed to international shipments of unprocessed logs (i.e. logs with bark) (May 1934). More recently, however, solid wood packaging material is considered the dominant invasion pathway for insects that feed beneath bark. Solid wood packaging material refers to crating, pallets, spools and dunnage (e.g. timbers used to prop up maritime cargo or containers). Increases in global movement of solid wood packaging material corresponds to the surge in the use of large container ships for cargo transport beginning in the 1980’s. Increased use of solid wood packaging material has resulted in a notable jump in the number of bark and wood-boring insects introduced to North America and elsewhere over the last few decades (Brockerhoff et al. 2006a; Aukema et al. 2010). Wood packaging material is typically made from relatively poor quality trees and low cost wood. Such wood is often infested with wood-boring insects. Many of these insects can survive for months, especially if some amount of bark remains on the wood to retain moisture. Insects in wood may complete development then emerge in a new habitat or region.
A substantial number of non-native forest insect species have been introduced intentionally, mostly for biological control of damaging invasive pests. In classical biological control, natural enemies from the native range of an invasive pest are imported, reared and released into the new habitat, ideally to provide long-term control of the invasive pest. If successful, such efforts can provide long-term control of a pest across a broad area. Insect parasitoids and predators are most often used in classical biocontrol programs, although in a few instances, a specialized pathogen may be considered.
Kenis et al. (2017) reported that worldwide, 6158 species of parasitoids or predators have been introduced for control of 588 forest insect pests and of those, 172 pest species were controlled with some success.
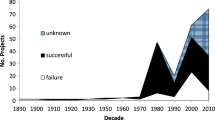
Historical rates of establishment of non-native insect species in various world regions (Fig. 23.1) indicate that the accumulation of non-native forest insects has not slowed during the last century (Brockerhoff and Liebhold 2017; Seebens et al. 2017). In fact, the rate of establishment may even be increasing in some regions, such as Europe. However, in other areas, numbers of new establishments per year of certain insect groups have declined. Such declines are sometimes a result of improved biosecurity practices (Liebhold and Griffin 2016). In other cases, declines may reflect the depletion of the supply of species capable of invading a specific new range (Levine and D’Antonio 2003). For example, numbers of bark beetle (Scolytinae) species invading N. America from Europe have declined, at least in part because centuries of trade between these continents have depleted the pool of European species capable of arriving and establishing in North America. In contrast, invasions of Asian bark beetle species have continued to increase. Substantial imports of commodities from Asia into N. America are comparatively recent and species pools have not yet been depleted (Liebhold et al. 2017b).
Numbers of new non-native forest insects discovered by decade in New Zealand, Europe and USA. Redrawn from Brockerhoff and Liebhold (2017)
Economic analyses (e.g. Leung et al. 2002, 2014; NRC 2002) consistently suggest that the most effective strategy for mitigating the biological invasion problem is prevention—i.e. taking measures to prevent the transport and arrival of non-native species. This is generally true for forest insect pests and there are several approaches for preventing their arrival. Most prevention strategies focus on managing the two dominant invasion pathways for forest insects: live plants and wood.
Importation of plants has historically played a crucial role in their domestication and genetic improvement for agricultural, forestry and ornamental purposes. Because imported plants obviously represent a high-risk invasion pathway for insects and plant pathogens, several measures have been identified to limit this risk. First, import of high-risk plant species can be simply banned. Some countries implement “black list” systems (e.g. imports of certain plant taxa are banned), while other countries use “white list” systems (plant taxa are banned unless they are known to be of relatively low risk). Although this is a somewhat simplistic representation, the latter system is considered more effective at preventing introductions of unknown organisms (Eschen et al. 2015). Second, phytosanitary treatments, such as fumigation or treatment of plants with pesticides, can reduce the likelihood that pests will be introduced on high-risk plants. Post-entry quarantines may also be applied. In this practice, imported plants are initially cultivated in a quarantine facility or secure location and monitored to ensure they are free of insects or pathogens before they are released for sale or cultivation.
“Integrated measures” can also be used to reduce risks of introducing new insect pests with imported plants. This involves applying multiple measures, often before the plants are shipped and again when the plants arrive. This approach can include phytosanitary treatments and inspections of plants before and after ship**. This may also include efforts to suppress pests at overseas plant production facilities. Insecticides or other pest management tactics may be used to ensure plant material is pest-free when it is exported (International Plant Protection Convention 2012).
Considerable variation exists among nations with regard to their regulation of plant imports (Eschen et al. 2015). New Zealand and Australia apply strict regulations based on a white list system, which limits the plant taxa that can be imported without phytosanitary treatments and/or post-entry quarantine. In contrast, the European Union implements relatively relaxed regulations. Some plants can be imported to Europe without any permit and in soil, which could harbor nematodes, plant pathogens and other pests. Regulations in other countries such as the USA, Canada and Japan, fall somewhere in between these extremes (Eschen et al. 2015). Such biosecurity measures have successfully reduced risks of pest introductions with legally imported plants (Liebhold and Griffin 2016). Illegal importation of plants, however, either by members of the public unaware of regulations or by importers deliberately avoiding oversight, represents a relatively uncontrolled invasion pathway. Many countries with develo** economies lack the resources to implement biosecurity practices. These areas potentially can serve as “bridgeheads” enabling alien species to become established and eventually invade other regions (Hurley et al. 2017).
Effectively regulating imports of wood to prevent insects and diseases from accidentally being transported remains challenging. Several countries ban imports of logs with bark, since many insects are associated with bark and phloem. Other countries allow logs to be imported but require fumigation either before or during international shipment (Allen et al. 2017). In 2002, the International Plant Protection Convention implemented a harmonized phytosanitary standard, ISPM 15 to reduce the risk of introducing live pests in solid wood packing material with imported cargo. The ISPM 15 standard requires heat treatment, fumigation or other measures be applied to solid wood packaging material used with cargo moving between countries. All countries that implemented ISPM 15 agreed to abide by these same regulations; hence the standard is “harmonized.” Data from cargo inspections at ports conducted by regulatory officials from 2003–2009 showed the implementation of ISPM 15 decreased rates of insect contamination in solid wood packaging material by 36–52% (Haack et al. 2014). Although 100% effectiveness of these treatments would be highly desirable, a cost–benefit analysis showed the economic costs of ISPM 15 were substantially lower than the economic benefits resulting from reducing rates of insect invasions (Leung 2014).
Although vast amounts of cargo arrive at many ports and border crossings, only a small fraction of any shipment can be inspected. Additionally, many insects and plant pathogens are small, cryptic and difficult to observe. Consequently, inspection may not be highly effective as a method of directly preventing arrival and introduction of unwanted organisms. It does, however, serve an important purpose as an incentive for producers and importers to reduce pest contamination of shipments and as a source of information about the species of pests associated with particular imports (Whattam et al. 2014). Inspections can also provide information about organisms that are relatively abundant in specific pathways, which may play a crucial role in identifying the need for new quarantine or phytosanitary measures (McCullough et al. 2006).
Inspection of air passengers to detect biosecurity threats occurs to varying degrees in different countries. Quarantine officers inspecting the baggage of air passengers arriving in the US have reported 10,000—20,000 interceptions of insects each year. These interceptions include insects from all orders and numerous species of concern to biosecurity (Liebhold et al. 2006). In New Zealand, quarantine officers inspect baggage and question all arriving passengers about items that could be infested with insects or other biosecurity threats. For example, several cam** tents carried by passengers were found to contain live insects (Gadgil and Flint 1983).
23.3 Establishment
Although many different species of non-native forest insects are transported across borders or through ports every year, only a small fraction of those species actually become established in the new region (NRC 2002; Blackburn et al. 2011). Generally, only a few “colonists” are transported to a new region on imported plants, wood or other materials. Upon arrival, this very small population must be able to survive the local climate, locate and successfully feed on a suitable host, and reproduce. Very low-density populations of a non-native insect face a high probability of extinction, much like native endangered species. When populations are at very low densities, they are especially vulnerable to unpredictable events that can lead to extinction. Such events, termed stochastic effects, can include unfavorable weather. Unusually cold temperatures in spring, a wildfire or a bad storm, for example, can wipe out a small, low-density population.
Allee effects (see Chapter 5) can also cause a newly or recently established population to go extinct (Lande 1998). Named after the University of Chicago professor Warder Allee who first described the phenomenon in 1949, the Allee effect refers to the phenomenon of decreasing population growth with decreasing density of an organism. In other words, very small populations of some species tend to become even smaller over time. For example, when spongy moth (Lymantria dispar) populations are at very low population densities, males may be unable to locate femalesfor mating. Low reproduction success causes the density to drop even further (Tobin et al. 2009). If the density of a population drops below a critical threshold level, the population will decline to extinction (Liebhold and Tobin 2008). Most populations of non-native insects arrive at densities below these thresholds, which helps to explain why so many fail to establish.
Several mechanisms can cause Allee effects in forest insect populations. Most insect species reproduce sexually and if densities are very low, may be unable to find a mate, leading to Allee dynamics (Gascoigne et al. 2009). Some species, including certain bark beetles, must mass attack their host tree to successfully overcome host defenses and reproduce within the tree. Such a phenomenon, a form of “group feeding,” is also capable of producing an Allee effect (Chase 2016). Additionally, attack by predators may create a weak Allee effect; predation levels are typically higher in small populations but with large populations there may be “safety in numbers” and therefore greater survival.
Given the assorted mechanisms that may cause Allee effects, plus the variation in their strength in affecting different species, it is not surprising that the probability of establishment varies considerably among insect species. Many species of Hemiptera, such as scales and adelgids, for example, reproduce asexually and population growth is not limited by the need to find mates. This life history trait may be one reason why Hemiptera are generally over-represented in non-native insect assemblages and relatively more successful invaders (Liebhold et al. 2016a). Aggressive tree-killing bark beetles such as the North American species, Dendroctonus ponderosae, and the European species, Ips typographus (Curculionidae), reach very high densities during outbreaks. Although both species have repeatedly been intercepted at overseas ports of entry, neither has become established outside of their native range. This likely reflects a strong Allee effect; density of the introduced populations is not high enough to enable the beetles to overcome resistance of their host trees and successfully reproduce (Brockerhoff et al. 2006a).
Management to prevent establishment of non-native populations plays a key role in biosecurity strategies. The general approach shared among all such efforts is a combination of surveillance, to find newly arrived reproducing populations, and eradication, the forced extinction of a population (Liebhold and Tobin 2008; Liebhold et al. 2016b). Techniques for surveillance of non-native insect populations may take a variety of forms, depending upon the biology and behavior of the target species (see Chapter 4) and the potential impacts of the species.
Detection surveys commonly rely on traps baited with lures containing pheromones or compounds produced and emitted into the air by host plants. If lures are highly attractive to the target pest, baited traps can be very sensitive tools and effectively detect low-density populations. For example, traps baited with synthetic sex pheromones are used in many countries for detecting newly arrived and very low-density populations of moths, such as the spongy moth. In contrast, traps baited with compounds produced by host trees, such as alpha-pinene, are widely used for detecting an array of conifer bark beetles, as well as phloem- and wood-boring insects (Brockerhoff et al. 2006b; Rabaglia et al. 2019). Traps baited with host volatiles are typically less sensitive than pheromone-baited traps. Often the lures with host volatiles do not strongly attract the target pest or the lures may be overwhelmed by complex blends of compounds produced by nearby live trees.
Some insects are not attracted to any type of chemical lure and other surveillance options, such as light traps or visual searches for evidence of infestation, may be the only option available (Chapter 19). Analysis of historical insect eradication programs indicates that the availability of a sensitive tool, such as attractant-baited traps, greatly increases the likelihood of early detection and successful eradication of invading populations (Tobin et al. 2014). Sensitive detection tools also provide an effective means to delimit (i.e. delineate spatial boundaries) invading populations and evaluate the success or failure of eradication programs.
Although eradication may be difficult or even impossible when there are no effective options to detect low-density populations or when a non-native population has already spread across a large area, there are many examples of forest insect species that have been successfully eradicated (Brockerhoff et al. 2010). Painted apple moth, Orgyia anartoides (Erebidae), an Australian Lymantriinae, was eradicated from New Zealand between 2001 and 2003 using a combination of tactics including host plant removal, aerial application of a microbial insecticide (Bacillus thuringiensis) and sterile male releases (Suckling et al. 2007) (Fig. 23.2). A remarkable aspect of this program is that pheromone-baited traps were not used in the eradication. Synthetic pheromone was found to be unstable and could not be used in lures. Instead, traps used for delimitation were baited with live female moths that were reared in the laboratory then placed in small cages attached to traps.
Scenes from a spongy moth eradication program in Washington, USA. A. Arial application of Bacillus thuriengensis; B. Fifth instar spongy moth larva; C. Public notification of aerial spraying; D. Checking traps to confirm eradication success [Photo credits: (A and C) James Marra, Washington State Department of Agriculture; (B) Jon Yuschock, Bugwood.org; (D) USDA APHIS PPQ, USDA APHIS PPQ, Bugwood.org]
In another example, an extensive population of Asian longhorned beetle (ALB), Anoplophora glabripennis (Cerambycidae), was successfully eradicated from Chicago (1998–2008) without the use of any traps or attractants for delimitation. Instead, delimitation was accomplished via visual surveys for characteristic holes on tree boles and branches left by emerging adult beetles. Eradication was accomplished by a combination of host removal and injections of systemic insecticides into all potential host trees within 400 m of positive finds (Haack et al. 2010). Other recent ALB eradication projects, such as those in Ontario and New Jersey, have relied strictly on removal of potential host trees within either a 400 m or 800 m radius of every infested tree (Turgeon et al. 2007). The success of these and other ALB eradication programs can be attributed, in part, to the limited dispersal behavior of beetles which constrained spread of the invading populations.
One of the challenges managers of surveillance and eradication strategies may face is the negative reaction to control activities that is sometimes expressed by residents of the treatment area. Invasions of forest insects characteristically occur in urban / suburban habitats (Poland and McCullough 2006) and residents may strongly oppose activities such as removal of apparently healthy (but possibly infested) trees or widespread pesticide applications (Liebhold et al. 2016b). Successful eradication programs, such as the painted apple moth eradication from Auckland, New Zealand and the ALB eradications in Chicago and Toronto, typically involve considerable effort in public engagement to explain the need for and value of such efforts. Other cases, such as the failed eradication of the light brown apple moth from California (Lindeman 2013; Suckling et al. 2014) demonstrate that public engagement and outreach efforts are essential to build support for the program. Organized opponents may otherwise step in and disseminate misinformation, eroding support for these programs.
23.4 Spread
Once a non-native population of a forest insect becomes established in a region, populations can build and typically begin expanding further into suitable habitats (Blackburn et al. 2011). This spread often occurs via two mechanisms; natural dispersal of the insects and accidental transport of insects by people. Natural dispersal can occur when insects fly or are transported by wind, birds or other animals. Long distance spread occurs when people move insect life stages or infested plant material into uninfested areas. Domestic invasion pathways refer to the means by which non-native forest insects are accidentally introduced into new states, provinces or currently uninfested regions. These domestic pathways often resemble those for intercontinental invasions. Human movement of live plants, and infested firewood, logs or solid wood packaging material may inadvertently transport non-native insects. Life stages of certain insect species can also hitchhike on non-host goods shipped from an infested area.
Rates of spread vary considerably among non-native forest insect species (Fig. 23.3). Spread of a non-native species represents the combination of population growth and dispersal; factors that affect either of these components will likely affect rates of spread (Liebhold and Tobin 2008). Rates of historical spread of invasive forest insects and diseases in the USA are positively related to human population density, host tree density and voltinism (Hudgins et al. 2017; Fahrner and Aukema 2018). Similarly, human population density is positively related to historical spread of the horse chestnut leafminer, Cameraria ohridella (Gracillariidae), in Europe (Gilbert et al. 2004).
One approach to managing spread involves regulating the pest and often the tree species or commodity that is likely to introduce the pest. For example, transport of ash trees from nurseries, ash logs and firewood were regulated in North America by federal and parallel state quarantines imposed to limit spread of the emerald ash borer (Herms and McCullough 2014). States, provinces or other regional governments may also impose their own quarantines to prevent the introduction of an invasive pest established in other regions. Quarantines typically prohibit transport of potentially infested host material unless specific phytosanitary treatments are applied to ensure the trees or wood is not infested.
Another approach to controlling spread involves using barrier zones to slow or stop the spread of an invading species. Perhaps the most extensive of such programs is the spongy moth ‘slow the spread’ program (Sharov et al. 2002) in the US. Each year, a grid of ca. 100,000 pheromone traps is deployed across a 100 km wide band along the leading edge of the spongy moth invasion in the US. When a new, isolated colony of spongy moth is detected, it is delimited, then treated to either eradicate the population or to dramatically reduce the density of the population. This approach has substantially slowed spongy moth spread in the US, yielding economic benefits by delaying establishment and thus impacts of this pest species (Epanchin-Niell and Liebhold 2015).
23.5 Established Populations
Ecological and economic impacts of non-native forest insect pests vary considerably ranging from minor defoliation to widespread mortality of host trees. Invasive forest insects that can kill their hosts are obviously of great concern. Emerald ash borer, first discovered in N. America in 2002, has become the most destructive and costly forest insect to invade that continent (Herms and McCullough 2014). This beetle has already killed hundreds of millions of ash (Fraxinus spp) in forests and landscapes in the US and Canada and continues to spread (Morin et al. 2017). Impacts of other invasive insects, such as hemlock woolly adelgid, Adelges tsugae (Adelgidae) and beech scale, Cryptococcus fagisuga (Eriococcidae), have resulted in large-scale shifts in forest composition, altering trajectories of regional forest and tree species succession (Morin and Liebhold 2015; Lovett et al. 2016).
In many parts of the world non-native tree species are planted for fiber production; much of the exceptional growth of such tree species can be attributed to their escape from insects and diseases present in their native ranges. However, these plantations can be particularly susceptible to invasions of insects and diseases from the native range of the tree species (Wingfield et al. 2015). In some cases, insect invasions have caused forest practices involving certain tree species to be totally abandoned (Hurley et al. 2016). For example, planting of Eucalyptus spp. in New Zealand was largely discontinued following the establishment of the eucalyptus tortoise beetle, Paropsis charybdis (Chrysomelidae), and other insects and pathogens from Australia (Withers 2001).
While invasive forest insects can have substantial impacts on market resources such as timber, the primary economic impacts of many of these species are largely in non-market economic sectors. Aukema et al. (2011) compiled a comprehensive analysis of economic costs associated with invasive phloem- and wood-boring, sap-feeding and foliage feeding insects in the US. The greatest economic impacts for all three feeding guilds were sustained by local governments, i.e. municipalities, and private property owners. These costs reflect the high value of trees growing in public and private landscapes, along roadways, and in parks or recreation areas. Property owners incur costs when trees must be protected from a pest with insecticides or when dead, dying or severely declining trees require removal (e.g. Kovacs et al. 2010). Dead and declining trees also reduce property values (Holmes et al. 2006). Additional costs are sustained because of the loss of ecological services provided by urban trees, such as storm water uptake and pollutant capture (Nowak et al. 2001; Jones 2017). Impacts of invasive forest insects on ecosystem services provided by forests can be complex and are not well understood (Boyd et al. 2013). Information to date indicates considerable variation in the impact of invasive insect pests on forest ecosystem processes such as nutrient cycling, and food web structure (Lovett et al. 2006).
What explains the often unusually high population growth rates that many introduced non-native forest insects exhibit following initial invasion? The answer may lie with evolutionary history. Most species evolved during millions of years, with natural selection sha** their interactions with other insect species and with their host trees. But when insects establish in alien habitats, the species with which they co-existed in their native range are typically absent. This suggests two mechanisms likely contribute to the often explosive population growth. First, the complex of natural enemies that regulate populations in their native range are typically absent in invaded regions. When a non-native forest insect species becomes established in the absence of predators, parasitoids and pathogens, populations may quickly grow to high levels (Colautti et al. 2004). Several foliage-feeding Lepidoptera represent examples of this ‘enemy release’ phenomenon. For example, the brown tail moth, Euproctis chrysorrhoea (Erebidae), which was introduced to North America in the late 1800’s, caused major defoliation of forests in the northeastern US until a generalist parasitoid, Compsilura concinnata (Tachinidae), was introduced in 1906. This Dipteran parasitoid is credited with causing the collapse of brown tail moth populations, including their virtual extinction over most of the invaded range (Elkinton et al. 2006). Unfortunately, this parasitoid, which is now well established across much of the US, also attacks many moth species native to North America and is credited with dramatic reductions in populations of native saturniid moths in the northeast (Elkinton and Boettner 2012).
Severe impacts by invasive forest insects in their new habitat often reflect a lack of host resistance. Most forest insects have co-evolved with the host trees they colonize in their native range for millions of years. Over time, trees usually have evolved at least some amount of resistance to insect herbivores, which acts to constrain population growth of these insect populations. When insects invade a new region, however, they are often able to feed and develop on tree species in the new habitat that are similar to their original hosts. However, without any previous evolutionary exposure, those tree species may lack resistance to the non-native insect, especially if there are no similar insect species in the invaded region. Insects that encounter such a “defense free space” may thus thrive at the expense of their novel host (Gandhi and Herms 2010). For example, emerald ash borer populations are native to China and other regions of Asia, where they act as secondary pests, colonizing Asian ash trees that are only stressed or dying. North American ash species however have no co-evolutionary history with emerald ash borer and healthy, as well as stressed, ash trees can be colonized and killed by this invader (Herms and McCullough 2014).
Novel associations between non-native forest insects and other non-host organisms, such as symbiotic fungi, can also lead to severe impacts, particularly by non-native bark beetles (Wingfield et al. 2017). Many bark beetles (Scolytinae) have important associations with fungi that they introduce into trees. In some tree species, certain mutualistic fungi improve nutrient levels or other conditions for the bark beetles (Six and Wingfield 2011). These mutualistic fungi may play a key role in determining whether a non-native bark beetle species can colonize healthy trees in a new region. As an example, the red turpentine beetle, Dendroctonus valens (Curculionidae), is native to North America where it acts as a secondary pest, colonizing stressed pine trees. Where it has invaded China, however, associations with novel Ophiostomatoid fungi enable the beetles to colonize healthy pines and thus act as a primary pest (Sun et al. 2013).
Understanding the mechanisms that contribute to severe impacts of invasive forest insects can help to identify possible strategies for managing a given pest. In the case of enemy release, classical biological control, which involves importing, rearing and releasing natural enemies from the pest’s native range, may be appropriate. Many of these efforts are not successful, but there are cases where an imported biological control agent has virtually eliminated outbreaks and damage caused by an invasive species (Kenis et al. 2017). For example, an egg parasitoid Anaphes nitens (Mymaridae), was imported and has provided effective control of the Eucalyptus snout beetle, Gonipterus spp. (Curculionidae), which was previously a serious defoliating pest of Eucalyptus in Africa, New Zealand, South America and California (Tribe 2005; Schröder et al. 2020). While classical biological control can reduce impacts of invasive forest insects over the long term, potential effects of imported natural enemies on native insects can create unintended problems (Hajek et al. 2016). Imported natural enemies may begin to prey on native non-target insects that cause little or no damage. They may also displace or outcompete native natural enemies that control native pests. Currently, biological control introductions are closely regulated in most countries. Ideally, potential biological control agents should be carefully screened to verify that they will attack the invasive target pest without adversely affecting native species.
When damage by an invasive forest insect is driven primarily by a lack of host resistance in the new region, selection for and breeding resistant trees may help reduce impacts. Different strategies, such as backcrossing susceptible native species with resistant species or genetic editing may be used to increase resistance to a particular pest (Sniezko and Koch 2017; Showalter et al. 2018). While selecting for or breeding host resistance holds promise, there are few examples where this has been successfully applied to overcome impacts of a damaging invasive forest insect. A major challenge with this strategy is deployment—i.e. establishing resistant trees in forests over large regions. While resistant genotypes can be cultivated and planted in ornamental settings or in forest plantations relatively easily, deployment of resistant strains is much more difficult in forests where natural regeneration dominates and competition with other species may be intense.
Today, chemical insecticides are rarely used to control invasive insect pests in forested settings because of an array of environmental and economic concerns. In urban forests, however, insecticides are frequently used to protect valuable landscape trees from an array of insect pests, including invasive species. Systemic products, which are applied by injecting the insecticide into the base of the trunk, pouring the insecticide around the base of the trunk, or spraying the lower portion of the trunk and allowing the insecticide to penetrate the outer bark, have largely replaced insecticide cover sprays. Systemic products greatly reduce insecticide impacts on beneficial insects and other non-target organisms, environmental residues and applicator exposure, and can effectively control insects feeding in phloem or in tree canopies (McCullough 2019). While these insecticides can obviously protect individual trees, in a few cases, systemic insecticides are applied to reduce the impacts of an invasive forest insect over large areas. In one large-scale project, a relatively small proportion of ash trees were treated with a systemic insecticide to successfully slow the rate of emerald ash borer population growth and the rate at which ash trees declined and died (Mercader et al. 2015, 2016). Many states in the eastern U.S. apply systemic insecticides to suppress hemlock woolly adelgid populations, protecting watersheds and riparian areas where hemlock trees are abundant (e.g. Benton et al. 2015). The bacterial pesticide Bacillus thuringiensis is sometimes applied aerially to suppress outbreak populations of foliage-feeding invasive pests such as the spongy moth, and thereby reduce impacts caused by defoliation (van Frankenhuyzen 2000).
Silviculture represents another strategy for reducing the impacts of damaging invasive forest insects (Muzika 2017). Silvicultural practices can increase stand level resistance to invasive, as well as native, forest insect pests. Increasing diversity at genetic, species and landscape scales, for example, will generally decrease susceptibility of forests to invasive, as well as native, insect pests. Diverse forests also provide habitat for an array of insect predators and parasitoids, which can often help control pest populations. Thinning and other practices to decrease competition and maintain healthy stands can contribute to reducing invasive pest impacts. For example, the invasion of Sirex noctilio (Siricidae) in New Zealand around 1900 resulted in high mortality in dense plantations of non-native pines. Over time, outbreaks of this invasive woodwasp generally subsided, probably as a result of the introduction of biological control agents (a nematode and two parasitoid species) plus an emphasis on thinning overstocked pine stands. Damage was much less severe in thinned forests than in dense stands, where competition reduced tree vigor (Hurley et al. 2007). While silvicultural practices may help to reduce damage caused by invasive forest insects that exploit low-vigor trees, there are often few options for pests that can colonize healthy trees aside from conversion of stands to favor non-host tree species.
23.6 Conclusions
Only a few decades ago, most of the important insect pests that forest entomologists focused on were native species. As the world has globalized, however, an ever-increasing proportion of the significant forest pests are invasive. Invasive forest insects have largely transformed the field of forest entomology and have changed our overall approach to forest pest problems. They have also greatly affected silvicultural management of stands dominated by affected tree species. Additionally, plantations of non-native trees are likely to play an increasing role in fulfilling the world’s demand for wood products in the future. Excluding invading pest species will play a critical role in maintaining the high productivity of these stands; hence forest biosecurity is likely to increase in importance (Wingfield et al. 2015).
There is little question that with current trends of globalization, insect species will continue to be introduced to new regions and some of these species will become established. Although the pool of species that could eventually become invasive is decreasing in some regions (Liebhold et al. 2017b), more invasions are inevitable, reflecting a combination of increased rates of imports, new trading partners and creation of new invasion pathways. These mechanisms, alone or collectively, can increase the exposure of one region to new, previously untapped species pools. Furthermore, there is often a considerable delay of 10–50 years between the establishment of a non-native species and its “discovery” when damage becomes extensive and readily apparent (Epanchin-Niell and Liebhold 2015). This means that many new but currently unknown species have probably already established. A portion of these species will inevitably emerge sometime in the future as serious problems.
Climate change, which is covered in Chapter 22, will affect future impacts of forest insects, including those resulting from invasive species. Range expansion or shifts, altered development rates, and changes in how forest insects interact with their hosts and natural enemies will undoubtedly be influenced by changing temperature and precipitation patterns in the future (e.g. Battisti et al. 2005).
References
Allen E, Noseworthy M, Ormsby M (2017) Phytosanitary measures to reduce the movement of forest pests with the international trade of wood products. Biol Invasions 19:3365–3376
Aukema JE, McCullough DG, Von Holle B, Liebhold AM, Britton K, Frankel SJ (2010) Historical accumulation of nonindigenous forest pests in the continental United States. Bioscience 60:886–897
Aukema JE, Leung B, Kovacs K, Chivers C, Britton KO, Englin J, Frankel SJ, Haight RG, Holmes TP, Liebhold AM, McCullough DG (2011) Economic impacts of non-native forest insects in the continental United States. PLoS One 6:e24587
Battisti A, Stastny M, Netherer S, Robinet C, Schopf A, Roques A, Larsson S (2005) Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecol Appl 15:2084–2096
Benton EP, Grant JF, Webster RJ, Nichols RJ, Cowles RS, Lagalante AF, Coots CI (2015) Assessment of imidacloprid and its metabolites in foliage of eastern hemlock multiple years following treatment for hemlock woolly adelgid, Adelges tsugae (Hemiptera: Adelgidae), in forested conditions. J Econ Entomol 108:2672–2682
Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarošík V, Wilson JRU, Richardson DM (2011) A proposed unified framework for biological invasions. Trends Ecol Evol 26:333–339
Boyd IL, Freer-Smith PH, Gilligan CA, Godfray HCJ (2013) The consequence of tree pests and diseases for ecosystem services. Science 342:1235773
Brockerhoff EG, Liebhold AM (2017) Ecology of forest insect invasions. Biol Invasions 19:3141–3159
Brockerhoff EG, Bain J, Kimberley M, Knížek M (2006a) Interception frequency of exotic bark and ambrosia beetles (Coleoptera: Scolytinae) and relationship with establishment in New Zealand and worldwide. Can J for Res 36:289–298
Brockerhoff EG, Jones DC, Kimberley MO, Suckling DM, Donaldson T (2006b) Nationwide survey for invasive wood-boring and bark beetles (Coleoptera) using traps baited with pheromones and kairomones. For Ecol Manage 228:234–240
Brockerhoff EG, Liebhold AM, Richardson B, Suckling DM (2010) Eradication of invasive forest insects: concepts, methods, costs and benefits. NZ J Forest Sci 40(Suppl):S117–S135
Chase KD (2016) Allee effects, host tree density and the establishment of invasive bark beetles. Doctoral Dissertation, Dept. of Biology, University of Canterbury. http://hdl.handle.net/10092/12581
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733
Elkinton JS, Parry D, Boettner GH, G.H, (2006) Implicating an introduced generalist parasitoid in the invasive browntail moth’s enigmatic demise. Ecology 87:2664–2672
Elkinton JS, Boettner GH (2012) Benefits and harm caused by the introduced generalist tachinid, Compsilura concinnata, in North America. Biocontrol 57:277–288
Epanchin-Niell RS, Liebhold AM (2015) Benefits of invasion prevention: effect of time lags, spread rates, and damage persistence. Ecol Econ 116:146–153
Eschen R, Britton K, Brockerhoff E, Burgess T, Dalley V, Epanchin-Niell RS, Gupta K, Hardy G, Huang Y, Kenis M, Kimani E (2015) International variation in phytosanitary legislation and regulations governing importation of plants for planting. Environ Sci Policy 51:228–237
Fahrner S, Aukema BH (2018) Correlates of spread rates for introduced insects. Glob Ecol Biogeogr. https://doi.org/10.1111/geb.12737
Gadgil PD, Flint TN (1983) Assessment of the risk of introduction of exotic forest insects and diseases with imported tents. N Z J for 28:58–67
Gandhi KJ, Herms DA (2010) Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol Invasions 12:389–405
Gascoigne J, Berec L, Gregory S, Courchamp F (2009) Dangerously few liaisons: a review of mate-finding Allee effects. Popul Ecol 51:355–372
Ghelardini LN, Luchi F, Pecori AL, Pepori R, Danti GD, Rocca P, Capretti PT, Ssantini A (2017) Ecology of invasive forest pathogens. Biol Invasions 19:3183–3200
Gilbert M, Grégoire JC, Freise JF, Heitland W (2004) Long-distance dispersal and human population density allow the prediction of invasive patterns in the horse chestnut leafminer Cameraria ohridella. J Anim Ecol 73:459–468
Hajek AE, Hurley BP, Kenis M, Garnas JR, Bush SJ, Wingfield MJ, van Lenteren JC, Cock MJW (2016) Exotic biological control agents: a solution or contribution to arthropod invasions? Biol Invasions 18:953–969
Haack RA, Hérard F, Sun J, Turgeon JJ (2010) Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: a worldwide perspective. Annu Rev Entomol 55:521–546
Haack RA, Britton KO, Brockerhoff EG, Cavey JF, Garrett LJ, Kimberley M, Lowenstein F, Nuding A, Olson LJ, Turner J, Vasilaky KN (2014) Effectiveness of the International Phytosanitary Standard ISPM No. 15 on reducing wood borer infestation rates in wood packaging material entering the United States. PLoS One 9:5, p e96611
Halldórsson G, Th Benedikz O, Eggertsson ES, Oddsdóttir, and Óskarsson H (2003) The impact of the green spruce aphid Elatobium abietinum (Walker) on long-term growth of Sitka spruce in Iceland. For Ecol Manage 181:281–287
Herms DA, McCullough DG (2014) The emerald ash borer invasion of North America: history, biology, ecology, impacts and management. Annu Rev Entomol 59:13–30
Holmes TP, Murphy EA, Bell KP (2006) Exotic forest insects and residential property values. Agric Resour Econ Rev 35:155–166
Houston DR (1994) Major new tree disease epidemics: beech bark disease. Annu Rev Phytopathol 32:75–87
Hudgins EJ, Liebhold AM, Leung B (2017) Predicting the spread of all invasive forest pests in the United States. Ecol Lett 20:426–435
Hurley BP, Slippers B, Wingfield MJ (2007) A comparison of control results for the alien invasive woodwasp, Sirex noctilio, in the southern hemisphere. Agric for Entomol 9:159–171
Hurley BP, Garnas J, Wingfield MJ, Branco M, Richardson DM, Slippers B (2016) Increasing numbers and intercontinental spread of invasive insects on eucalypts. Biol Invasions 18:921–933
Hurley BP, Slippers B, Sathyapala S, Wingfield MJ (2017) Challenges to planted forest health in develo** economies. Biol Invasions 19:3273–3285
International Plant Protection Convention. (2012). ISPM-36, Integrated measures for plants for planting. United Nations food and agriculture organization http://www.fao.org/3/a-k8114e.pdf
Jones BA (2017) Invasive species impacts on human well-being using the life satisfaction index. Ecol Econ 134:250–257
Kenis M, Hurley BP, Hajek AE, Cock MJ (2017) Classical biological control of insect pests of trees: facts and figures. Biol Invasions 19:3401–3417
Kiritani K, Yamamura K (2003) Exotic insects and their pathways for invasion. In: Ruiz GM, Carlton JT (eds) Invasive species—vectors and management strategies. Island Press, Washington, pp 44–67
Kovacs KF, Haight RG, McCullough DG, Mercader RJ, Siegert NW, Liebhold AM (2010) Cost of potential emerald ash borer damage in US communities, 2009–2019. Ecol Econ 69:569–578
Lande R (1998) Demographic stochasticity and Alle effect on a scale with isotropic noise. Oikos 83:353–358
Leung B, Lodge DM, Finnoff D, Shogren JF, Lewis MA, Lamberti G (2002) An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive species. Proceedings of the Royal Society of London: Biological Sciences 269:2407–2413
Leung B, Springborn MR, Turner JA, Brockerhoff EG (2014) Pathway-level risk analysis: the net present value of an invasive species policy in the US. Front Ecol Environ 12:273–279
Levine JM, D’Antonio CM, CM (2003) Forecasting biological invasions with increasing international trade. Conserv Biol 17:322–326
Liebhold AM, Griffin RL (2016) The legacy of Charles Marlatt and efforts to limit plant pest invasions. Bull Entomol Soc Am 62:218–227
Liebhold AM, Tobin PC (2008) Population ecology of insect invasions and their management. Annu Rev Entomol 53:387–408
Liebhold AM, Macdonald WL, Bergdahl D, Mastro VC (1995) Invasion by exotic forest pests: a threat to forest ecosystems. For Sci Monogr 30:1–49
Liebhold AM, Work TT, McCullough DG, Cavey JF (2006) Airline baggage as a pathway for alien insect species invading the United States. Am Entomol 52:48–54
Liebhold AM, Brockerhoff EG, Garrett LJ, Parke JL, Britton KO (2012) Live plant imports: the major pathway for forest insect and pathogen invasions of the United States. Front Ecol Environ 10:135–143
Liebhold AM, Yamanaka T, Roques A, Augustin S, Chown SL, Brockerhoff EG, Pyšek P (2016a) Global compositional variation among native and non-native regional insect assemblages emphasizes the importance of pathways. Biol Invasions 18:893–905
Liebhold AM, Berec L, Brockerhoff EG, Epanchin-Niell RS, Hastings A, Herms DA, Kean JM, McCullough DG, Suckling DM, Tobin PC, Yamanaka T (2016b) Eradication of invading insect populations: from concepts to applications. Annu Rev Entomol 61:335–352
Liebhold AM, Brockerhoff EG, Kalisz S, Nuñez MA, Wardle DA, Wingfield MJ (2017a) Biological invasions in forest ecosystems. Biol Invasions 19:3437–3458
Liebhold AM, Brockerhoff EG, Kimberley M (2017b) Depletion of heterogeneous source species pools predicts future invasion rates. J Appl Ecol 54:1968–1977
Lindeman N (2013) Subjectivized knowledge and grassroots advocacy: an analysis of an environmental controversy in Northern California. J Bus Tech Commun 27:62–90
Lovett GM, Canham CD, Arthur MA, Weathers KC, Fitzhugh RD (2006) Forest ecosystem responses to exotic pests and pathogens in eastern North America. Bioscience 56:395–405
Lovett GM, Weiss M, Liebhold AM, Holmes TP, Leung B, Lambert KF, Orwig DA, Campbell FT, Rosenthal J, McCullough DG, Wildova R (2016) Nonnative forest insects and pathogens in the United States: impacts and policy options. Ecol Appl 26:1437–1455
May C (1934) Outbreaks of the Dutch elm disease in the United States. Circular 322, US Department of Agriculture, Washington, D.C., USA
McCullough DG, Work TT, Cavey JF, Liebhol AM, Marshall D (2006) Interceptions of nonindigenous plant pests at US ports of entry and border crossings over a 17-year period. Biol Invasions 8:611–630
McCullough DG (2019) Challenges, tactics and integrated management of emerald ash borer. Forestry: an International Journal of Forest Research. Forestry 93:197–211
Mercader RJ, McCullough DG, Storer AJ, Bedford J, Poland TM, Katovich S (2015) Evaluation of the potential use of a systemic insecticide and girdled trees in area wide management of the emerald ash borer. For Ecol Manage 350:70–80
Mercader RJ, McCullough DG, Storer AJ, Bedford JM, Heyd R, Siegert NW, Katovich S, Poland TM (2016) Estimating local spread of recently established emerald ash borer, Agrilus planipennis, infestations and the potential to influence it with a systemic insecticide and girdled ash trees. For Ecol Manage 366:87–97
Meurisse N, Rassati D, Hurley BP, Brockerhoff EG, Haack RA (2019) Common pathways by which non-native forest insects move internationally and domestically. J Pest Sci 92:13–27
Morin RS, Liebhold AM (2015) Invasions by two non-native insects alter regional forest species composition and successional trajectories. For Ecol Manage 341:67–74
Morin RS, Liebhold AM, Pugh SA, Crocker SJ (2017) Regional assessment of emerald ash borer, Agrilus planipennis, impacts in forests of the Eastern United States. Biol Invasions 19:703–711
Muzika RM (2017) Opportunities for silviculture in management and restoration of forests affected by invasive species. Biol Invasions 19:1–17
National Research Council, Board of Agriculture and Natural Resources, National Academy of Science (2002) Predicting invasions of nonindigenous plants and plant pests. National Academy Press, Washington, DC, p 198
Niemelä P, Mattson WJ (1996) Invasion of North American forests by European phytophagous insects. Bioscience 46:741–753
Nowak DJ, Pasek JE, Sequeira RA, Crane DE, Mastro VC (2001) Potential effect of Afzoplophoru glabripennis (Coleoptera: Cerambycidae) on urban tree in the United States. J Econ Entomol 94:116–122
Paine TD, Steinbauer MJ, Lawson SA (2011) Native and exotic pests of Eucalyptus: a worldwide perspective. Annu Rev Entomol 56:181–201
Poland TM, McCullough DG (2006) Emerald ash borer: invasion of the urban forest and the threat to North America’s ash resource. J Forest 104:118–124
Rabaglia RJ, Cognato AI, Hoebeke ER, Johnson CW, LaBonte JR, Carter ME, Vlach JJ (2019) Early detection and rapid response: a 10-Year summary of the USDA forest service program of surveillance for non-native bark and ambrosia beetles. Am Entomol 65:29–42
Roques A, Rabitsch W, Rasplus JY, Lopez-Vaamonde C, Nentwig W, Kenis M (2009) In: Nentwig W, Hulme P, Pysek P, Vila M (eds) Handbook of alien species in Europe. Springer, Dordrecht, pp 63–79
Showalter DN, Raffa KF, Sniezko RA, Herms DA, Liebhold AM, Smith JA, Bonello P (2018) Strategic development of tree resistance against forest pathogen and insect invasions in defense-free space. Front Ecol Evol 6:124
Schröder ML, Slippers B, Wingfield MJ, Hurley BP (2020) Invasion history and management of Eucalyptus snout beetles in the Gonipterus scutellatus species complex. J Pest Sci 93:11–25
Seebens H, Blackburn TM, Dyer EE, Genovesi P, Hulme PE, Jeschke JM, Pagad S et al (2017) No saturation in the accumulation of alien species worldwide. Nat Commun 8:14435
Sharov AAD, Leonard AM, Liebhold EA, Roberts WD (2002) “Slow The Spread”: A National Program to Contain the Gypsy Moth. J Forest 100:30–36
Six DL, Wingfield MJ (2011) The role of phytopathogenicity in bark beetle–fungus symbioses: a challenge to the classic paradigm. Annu Rev Entomol 56:255–272
Slippers B, Hurley BP, Wingfield MJ (2015) Sirex woodwasp: a model for evolving management paradigms of invasive forest pests. Annu Rev Entomol 60:601–619
Sniezko RA, Koch J (2017) Breeding trees resistant to insects and diseases: putting theory into application. Biol Invasions 19:3377–3400
Straw NA, Bellett-Travers M (2004) Impact and management of the horse chestnut leaf-miner (Cameraria ohridella). Arboric J 28:67–83
Suckling DM, Barrington AM, Chhagan A, Stephens AEA, Burnip GM, Charles JG, Wee SL (2007) Eradication of the Australian painted apple moth Teia anartoides in New Zealand: trap**, inherited sterility, and male competitiveness. In: Vreysen MJB, Robinson AS, Hendrichs J (eds) Area-wide control of insect pests. Springer, Dordrecht, pp 603–615
Suckling DM, Stringer LD, Baird DB, Butler RC, Sullivan TES, Lance DR, Simmons GS (2014) Light brown apple moth (Epiphyas postvittana) (Lepidoptera: Tortricidae) colonization of California. Biol Invasions 16:1851–1863
Sun J, Lu M, Gillette NE, Wingfield MJ (2013) Red turpentine beetle: innocuous native becomes invasive tree killer in China. Annu Rev Entomol 58:293–311
Tobin PC, Robinet C, Johnson DM, Whitmire SL, Bjørnstad ON, Liebhold AM (2009) The role of Allee effects in gypsy moth, Lymantria dispar (L.), invasions. Popul Ecol 51:373–384
Tobin PC, Kean JM, Suckling DM, McCullough DG, Herms DA, Stringer LD (2014) Determinants of successful arthropod eradication programs. Biol Invasions 16:401–414
Tribe GD (2005) The present status of Anaphes nitens (Hymenoptera: Mymaridae), an egg parasitoid of the Eucalyptus snout beetle Gonipterus scutellatus, in the Western Cape Province of South Africa. South Afr for J 203:49–54
Turgeon, J.J., J. Ric, P. de Groot, B. Gasman, M. Orr, J. Doyle, M. T. Smith, L. Dumouchel and T. Scarr. 2007. Détection des signes et des symptômes d’attaque par le longicorne étoilé: guide de formation. Service canadien des forêts, Ressources naturelles Canada, Ottawa, Ont.
van Frankenhuyzen K (2000) Application of Bacillus thuringiensis in forestry. In: Charles JF, Delécluse A, Roux CNL (eds) Entomopathogenic bacteria: from laboratory to field application. Springer, Dordrecht, pp 371–382
Whattam M, Clover G, Firko M, Kalaris T (2014) The biosecurity continuum and trade: border operations. In: Gordh G, McKirdy S (eds) The Handbook of Plant Biosecurity. Springer, Dordrecht, pp 149–188
Withers TM (2001) Colonization of eucalypts in New Zealand by Australian insects. Austral Ecol 26:467–476
Wingfield MJ, Brockerhoff EG, Wingfield BD, Slippers B (2015) Planted forest health: the need for a global strategy. Science 349:832–836
Wingfield MJ, Barnes I, de Beer ZW, Roux J, Wingfield BD, Taerum SJ, S.J. (2017) Novel associations between ophiostomatoid fungi, insects and tree hosts: current status—future prospects. Biol Invasions 19:3215–3228
Xu T, Hiroe Y, Teale SA, Fujiwara-Tsujii N, Wickham JD, Fukaya M, Hansen L et al (2017) Identification of a male-produced sex-aggregation pheromone for a highly invasive cerambycid beetle. Aromia Bungii. Sci Rep 7:7330
Acknowledgements
We thank E. Luzader for assistance with figures. A. Liebhold acknowledges support from the USDA Forest Service, the Swedish Agricultural University and grant “EVA4.0”, No. CZ.02.1.01/0.0/0.0/16_019/0000803 financed by OP RDE. This publication was supported in part by the HOMED project (http://homed-project.eu/), which received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 771271. Contributions by E.G.B. were also supported by the New Zealand government via MBIE core funding to Scion under contract C04X1104 and the Better Border Biosecurity Collaboration (www.b3nz.org).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Liebhold, A.M., Brockerhoff, E.G., McCullough, D.G. (2023). Forest Insect Invasions and Their Management. In: D. Allison, J., Paine, T.D., Slippers, B., Wingfield, M.J. (eds) Forest Entomology and Pathology. Springer, Cham. https://doi.org/10.1007/978-3-031-11553-0_23
Download citation
DOI: https://doi.org/10.1007/978-3-031-11553-0_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-11552-3
Online ISBN: 978-3-031-11553-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)