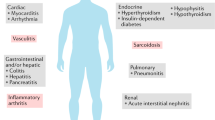
Abstract
Rheumatic immune-related adverse events (irAEs) associated with immune checkpoint inhibitor (ICI) therapy in patients with cancer can be grouped into articular, muscular, granulomatous, vasculitic, and other systemic irAEs. In a large registry study, the two most prevalent types of rheumatic irAEs were articular (36% of cases) and muscular (34%) [1]; these two types are the focus of this chapter. The articular cluster is comprised of arthralgias and arthritis, and the muscular cluster is comprised of myalgias, myositis, and polymyalgia rheumatica (PMR)-like syndrome. Complete resolution of arthritic and myositis symptoms is expected in most patients after the discontinuation of ICI and often a course of treatment with immunosuppression. However, persistent inflammatory arthritis that requires prolonged DMARDs (disease-modifying antirheumatic drugs) has been reported.
Chapter debriefing: PowerPoint video presentation is provided at the end of the chapter.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- ANA:
-
Anti-nuclear antibody
- CK:
-
Creatine kinase
- CRP:
-
C-reactive protein
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- CTLA- 4:
-
Cytotoxic T-lymphocyte-associated protein 4
- DMARD:
-
Disease-modifying antirheumatic drug
- GCA:
-
Giant cell arteritis
- ICI:
-
Immune checkpoint inhibitor
- irAE:
-
Immune-related adverse events
- PD-1:
-
Programmed cell death-protein1
- PD-L1:
-
Programmed death-ligand 1
- PMR:
-
Polymyalgia rheumatica
- RA:
-
Rheumatoid arthritis
- ReA:
-
Reactive arthritis
- RS3PE:
-
Remitting seronegative symmetrical synovitis with pitting edema
References
Ramos-Casals M, Lambotte O, Kostine M, Calabrese L, Suarez-Almazor M, Bingham C, Radstake TR, Baldini C, Schaeverbeke T, Gottenberg J-E. THU0649 phenotypic clusters of rheumatic/systemic immune-related adverse events induced by cancer immunotherapies (Immunocancer International Registry). BMJ Publishing Group Ltd; 2019.
Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol. 2018;14(10):569–79.
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32.
Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17.
Buder-Bakhaya K, Benesova K, Schulz C, Anwar H, Dimitrakopoulou-Strauss A, Weber TF, Enk A, Lorenz HM, Hassel JC. Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer Immunol Immunother. 2018;67(2):175–82.
Kostine M, Rouxel L, Barnetche T, Veillon R, Martin F, Dutriaux C, Dousset L, Pham-Ledard A, Prey S, Beylot-Barry M, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis. 2018;77(3):393–8.
Pundole X, Abdel-Wahab N, Suarez-Almazor ME. Arthritis risk with immune checkpoint inhibitor therapy for cancer. Curr Opin Rheumatol. 2019;31(3):293–9.
Cappelli LC, Brahmer JR, Forde PM, Le DT, Lipson EJ, Naidoo J, Zheng L, Bingham CO 3rd, Shah AA. Clinical presentation of immune checkpoint inhibitor-induced inflammatory arthritis differs by immunotherapy regimen. Semin Arthritis Rheum. 2018;48(3):553–7.
Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–86.
Leipe J, Christ LA, Arnoldi AP, Mille E, Berger F, Heppt M, Goldscheider I, Kauffmann-Guerrero D, Huber RM, Dechant C, et al. Characteristics and treatment of new-onset arthritis after checkpoint inhibitor therapy. RMD Open. 2018;4(2):e000714.
Braaten TJ, Brahmer JR, Forde PM, Le D, Lipson EJ, Naidoo J, Schollenberger M, Zheng L, Bingham Iii CO, Shah AA, et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis. 2020;79(3):332–8.
Cappelli LC, Gutierrez AK, Baer AN, Albayda J, Manno RL, Haque U, Lipson EJ, Bleich KB, Shah AA, Naidoo J, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2017;76(1):43–50.
Sang T. Kim, Yanshuo Chu, Mercy Misoi, Maria E. Suarez-Almazor, Jean H. Tayar, Huifang Lu, Maryam Buni, Jordan Kramer, Emma Rodriguez, Zulekha Hussain, Sattva S. Neelapu, Jennifer Wang, Amishi Y. Shah, Nizar M. Tannir, Matthew T. Campbell, Don L. Gibbons, Tina Cascone, Charles Lu, George R. Blumenschein, Mehmet Altan, Bora Lim, Vincente Valero, Monica E. Loghin, Janet Tu, Shannon N. Westin, Aung Naing, Guillermo Garcia-Manero, Noha Abdel-Wahab, Hussein A. Tawbi, Patrick Hwu, Isabella C. Glitza Oliva, Michael A. Davies, Sapna P. Patel, Jun Zou, Andrew Futreal, Adi Diab, Linghua Wang & Roza Nurieva. Distinct molecular and immune hallmarks of inflammatory arthritis induced by immune checkpoint inhibitors for cancer therapy. Nature communications 13.1 (2022):1–19.
Albayda J, Dein E, Shah AA, Bingham CO 3rd, Cappelli L. Sonographic findings in inflammatory arthritis secondary to immune checkpoint inhibition: a case series. ACR Open Rheumatol. 2019;1(5):303–7.
Subedi A, Williams SG, Yao L, Maharjan S, Strauss J, Sharon E, Thomas A, Apolo AB, Gourh P, Hasni SA. Use of magnetic resonance imaging to identify immune checkpoint inhibitor–induced inflammatory arthritis. JAMA Netw Open. 2020;3(2):e200032.
Cappelli LC, Grieb SM, Shah AA, Bingham CO, Orbai A-M. Immune checkpoint inhibitor-induced inflammatory arthritis: a qualitative study identifying unmet patient needs and care gaps. BMC Rheumatol. 2020;4(1):1–10.
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–68.
Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M, Dunnington D, et al. Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Cancer Netw. 2019;17(3):255–89.
Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95.
Kostine M, Finckh A, Bingham CO, Visser K, Leipe J, Schulze-Koops H, Choy EH, Benesova K, Radstake TR, Cope AP. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis. 2021;80(1):36–48.
Kim ST, Tayar J, Suarez-Almazor M, Garcia S, Hwu P, Johnson DH, Uemura M, Diab A. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis. 2017;76(12):2061–4.
Tsukamoto H, Fujieda K, Miyashita A, Fukushima S, Ikeda T, Kubo Y, Senju S, Ihn H, Nishimura Y, Oshiumi H. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78(17):5011–22.
Cappelli LC, Bingham CO. Expert perspective: immune checkpoint inhibitors and rheumatologic complications. Arthritis Rheumatol. 2021;73(4):553–65.
Zhong H, Zhou J, Xu D, Zeng X. Rheumatic immune-related adverse events induced by immune checkpoint inhibitors. Asia Pac J Clin Oncol. 2021;17(3):178–85.
Belkhir R, Le Burel S, Lambotte O, Mouterde G, Pertuiset E, Dunogeant L, Marabelle A, Leary A, Voisin A, Mariette X. OP0004 Rheumatoid arthritis occuring after immune checkpoint inhibitors. BMJ Publishing Group Ltd; 2017.
Jatwani K, Kaur H, Chugh K, Jatwani S. Nivolumab-induced psoriatic arthritis in a patient with advanced small cell lung cancer. J Clin Rheumatol. 2021;27(5):e162–3.
Nigro O, Pinotti G, Gueli R, Grigioni E, Santis M, Ceribelli A, Selmi C. Psoriatic arthritis induced by anti-PD1 and treated with apremilast: a case report and review of the literature. Immunotherapy. 2020;12(8):549–54.
Ghosh N, Tiongson MD, Stewart C, Chan KK, Jivanelli B, Cappelli L, Bass AR. Checkpoint inhibitor-associated arthritis: a systematic review of case reports and case series. J Clin Rheumatol. 2021;27(8):e317–22.
Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM: Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. 2017.
Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, Cohen J, Sullivan RJ. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124(18):3706–14.
Scott SC, Pennell NA. Early use of systemic corticosteroids in patients with advanced NSCLC treated with nivolumab. J Thorac Oncol. 2018;13(11):1771–5.
Abdel-Wahab N, Suarez-Almazor ME. Frequency and distribution of various rheumatic disorders associated with checkpoint inhibitor therapy. Rheumatology. 2019;58(Supplement_7):vii40–8.
Ramos-Casals M, Lambotte O, Kostine M, Calabrese L, Suarez-Almazor M, Bingham C, Radstake TR, Baldini C, Schaeverbeke T, Gottenberg J-E. Thu0628 immune-related adverse events induced by cancer immunotherapies. Big data analysis of 13,051 cases (Immunocancer international registry). BMJ Publishing Group Ltd; 2019.
Anquetil C, Salem J-E, Lebrun-Vignes B, Johnson DB, Mammen AL, Stenzel W, Léonard-Louis S, Benveniste O, Moslehi JJ, Allenbach Y. Immune checkpoint inhibitor–associated myositis: expanding the spectrum of cardiac complications of the immunotherapy revolution. Circulation. 2018;138(7):743–5.
Aldrich J, Pundole X, Tummala S, Palaskas N, Andersen CR, Shoukier M, Abdel-Wahab N, Deswal A, Suarez-Almazor ME. Inflammatory myositis in cancer patients receiving immune checkpoint inhibitors. Arthritis Rheumatol. 2021;73(5):866–74.
Shah M, Tayar JH, Abdel-Wahab N, Suarez-Almazor ME. Myositis as an adverse event of immune checkpoint blockade for cancer therapy. In: Seminars in arthritis and rheumatism: 2019. Elsevier; 2019. p. 736–40.
Kostine M, Truchetet M-E, Schaeverbeke T. Clinical characteristics of rheumatic syndromes associated with checkpoint inhibitors therapy. Rheumatology. 2019;58(Supplement_7):vii68–74.
Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, Lambotte O, Mariette X, Prat A, Suárez-Almazor ME. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):1–21.
Moreira A, Loquai C, Pföhler C, Kähler KC, Knauss S, Heppt MV, Gutzmer R, Dimitriou F, Meier F, Mitzel-Rink H. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur J Cancer. 2019;106:12–23.
Messer A, Drozd B, Glitza IC, Lu H, Patel AB. Dermatomyositis associated with nivolumab therapy for melanoma: a case report and review of the literature. Dermatol Online J. 2020;26(8):13030/qt4c21b068.
Bilen MA, Subudhi SK, Gao J, Tannir NM, Tu S-M, Sharma P. Acute rhabdomyolysis with severe polymyositis following ipilimumab-nivolumab treatment in a cancer patient with elevated anti-striated muscle antibody. J Immunother Cancer. 2016;4(1):1–5.
Fazel M, Jedlowski PM. Severe myositis, myocarditis, and myasthenia gravis with elevated anti-striated muscle antibody following single dose of ipilimumab-nivolumab therapy in a patient with metastatic melanoma. Case Rep Immunol. 2019;2019:2539493.
Suarez-Almazor ME, Calabrese LH. Rheumatic diseases and syndromes induced by cancer immunotherapy: a handbook for diagnosis and management. Springer Nature; 2020.
Mooradian MJ, Nasrallah M, Gainor JF, Reynolds KL, Cohen JV, Lawrence DP, Miloslavsky EM, Kohler MJ, Sullivan RJ, Schoenfeld SR. Musculoskeletal rheumatic complications of immune checkpoint inhibitor therapy: a single center experience. In: Seminars in arthritis and rheumatism: 2019. Elsevier; 2019. p. 1127–32.
Touat M, Maisonobe T, Knauss S, Salem OBH, Hervier B, Auré K, Szwebel T-A, Kramkimel N, Lethrosne C, Bruch J-F. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. 2018;91(10):e985–94.
Mammen AL, Rajan A, Pak K, Lehky T, Casciola-Rosen L, Donahue RN, Lepone LM, Zekeridou A, Pittock SJ, Hassan R. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann Rheum Dis. 2019;78(1):150–2.
Pourhassan HZ, Tryon D, Schaeffer B, Mirshahidi H, Wong J. Autoimmune rhabdomyolysis and a multiorgan display of PD-1 inhibitor induced immune related adverse events during treatment of metastatic melanoma. Exp Hematol Oncol. 2019;8(1):1–6.
Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–55.
Le Burel S, Champiat S, Mateus C, Marabelle A, Michot J-M, Robert C, Belkhir R, Soria J-C, Laghouati S, Voisin A-L. Prevalence of immune-related systemic adverse events in patients treated with anti-programmed cell death 1/anti-programmed cell death-ligand 1 agents: a single-centre pharmacovigilance database analysis. Eur J Cancer. 2017;82:34–44.
Calabrese C, Cappelli LC, Kostine M, Kirchner E, Braaten T, Calabrese L. Polymyalgia rheumatica-like syndrome from checkpoint inhibitor therapy: case series and systematic review of the literature. RMD Open. 2019;5(1):e000906.
Goldstein BL, Gedmintas L, Todd DJ. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of ctla-4. Arthritis Rheumatol. 2014;66(3):768–9.
Calabrese C, Kirchner E, Kontzias A, Velcheti V, Calabrese L. Rheumatic immune-related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD Open. 2017;3(1):e000412.
Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med. 2002;347(4):261–71.
Conflict of Interest
None of the authors have any conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Electronic Supplementary Material
Data 15.1
(PPTX 66273 kb)
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kim, S.T., Bowman, S., Lu, H. (2022). Rheumatology (Arthritis and Myositis). In: Wang, Y. (eds) Managing Immunotherapy Related Organ Toxicities. Springer, Cham. https://doi.org/10.1007/978-3-031-00241-0_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-00241-0_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-00240-3
Online ISBN: 978-3-031-00241-0
eBook Packages: MedicineMedicine (R0)