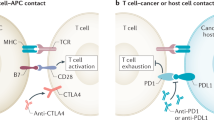
Abstract
Understanding of the mechanisms by which cancer cells evade T-cell immunity through interaction with a number of ligands has led to the introduction of immunotherapy. Immune checkpoint inhibitors prevent T-cell deactivation by malignant cells enhancing the anticancer immune response. Agents acting on CTLA-4 and PD-1, and its ligand are the principal therapeutic immunotherapy drugs in practice. These agents are potent and are now used in a variety of malignancies. Activation of the immune system results in risk of adverse effects, particularly affecting the skin, GI tract, and endocrine system. This review addresses the endocrine toxicities seen with immunotherapy. Thyroid, pituitary, and adrenal diseases are most common. Diabetes, as a result of islet-cell destruction, also occurs as do rarer endocrinopathies. Differences in rates and patterns of endocrine complications are seen with the CTLA-4 and PD-1 acting agents. This chapter summarizes the endocrine consequences of immunotherapy and provides guidance on the management of these common conditions.
Similar content being viewed by others
Abbreviations
- Ab:
-
Antibody
- ACTH:
-
Adrenocorticotropic hormone
- ADH:
-
Antidiuretic hormone
- APS:
-
Autoimmune polyendocrine syndrome
- BMI:
-
Body mass index
- CaSR:
-
Calcium sensing receptor
- CD8:
-
Cluster of differentiation 8
- CT:
-
Computed tomography
- CTCAE:
-
Cancer Institute Common Terminology Criteria for Adverse Events
- CTLA-4:
-
Cytotoxic T-lymphocyte antigen 4
- DKA:
-
Diabetic ketoacidosis
- DM:
-
Diabetes mellitus
- FDG-PET:
-
Fluorodeoxyglucose positron emission tomography
- FSH:
-
Follicle-stimulating hormone
- GAD:
-
Glutamic acid decarboxylase
- GLP1:
-
Glucagon-like peptide-1
- ICI:
-
Immune checkpoint inhibitor
- IFNɤ:
-
Interferon gamma
- IGF-1:
-
Insulin-like growth factor-1
- IgG:
-
Immunoglobulin G
- IL10:
-
Interleukin 10
- IL2:
-
Interleukin 2
- iRAE:
-
Immune-related adverse event
- IV:
-
Intravenous
- LH:
-
Luteinizing hormone
- MDI:
-
Multiple daily injections
- MRI:
-
Magnetic resonance imaging
- PAI:
-
Primary adrenal insufficiency
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed cell death protein ligand 1
- PTH:
-
Parathyroid hormone
- SUV:
-
Standardized uptake value
- TDS:
-
Ter die sumendum (to be taken three times daily)
- TGB:
-
Thyroglobulin
- TKI:
-
Tyrosine kinase inhibitor
- TME:
-
Tumor microenvironment
- TPO:
-
Thyroid peroxidase
- TSH:
-
Thyroid-stimulating hormone
- TSI:
-
Thyroid-stimulating immunoglobulin
- USS:
-
Ultrasound scan
References
Agarwala SS (2009) Current systemic therapy for metastatic melanoma. Expert Rev Anticancer Ther 9(5):587–595
Al Ashi SI, Thapa B, Flores M, Ahmed R, Rahim SEG, Amir M et al (2021) Endocrine toxicity and outcomes in patients with metastatic malignancies treated with immune checkpoint inhibitors. J Endocr Soc 5(8):bvab100
Albarel F, Gaudy C, Castinetti F, Carré T, Morange I, Conte-Devolx B et al (2015) Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol 172(2):195–204
Albarel F, Castinetti F, Brue T (2019) Management of endocrine disease: immune check point inhibitors-induced hypophysitis. Eur J Endocrinol 181(3):R107–RR18
Amereller F, Deutschbein T, Joshi M, Schopohl J, Schilbach K, Detomas M et al (2022) Differences between immunotherapy-induced and primary hypophysitis-a multicenter retrospective study. Pituitary 25(1):152–158
Anderson B, Morganstein DL (2021a) Endocrine toxicity of cancer immunotherapy: clinical challenges. Endocr Connect 10(3):R116–RR24
Anderson B, Morganstein DL (2021b) Endocrine toxicity of cancer immunotherapy: clinical challenges. Endocr Connect 10:R116–R124
Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H et al (2003) The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 198(1):63–69
Bai X, Chen X, Wu X, Huang Y, Zhuang Y, Lin X (2020a) Immune checkpoint inhibitor-associated thyroid dysfunction: a disproportionality analysis using the WHO adverse drug reaction database. VigiBase Eur J Endocrinol 182(1):1–9
Bai X, Chen X, Wu X, Huang Y, Zhuang Y, Chen Y et al (2020b) Immune checkpoint inhibitor-associated pituitary adverse events: an observational, retrospective, disproportionality study. J Endocrinol Investig 43(10):1473–1483
Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE et al (2018) Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 4(2):173–182
Bastin M, Busieau P, Kuhn E, Rouault C, Taboureau O, Toulgoat A et al (2021) Incretin response in immune checkpoint inhibitor-induced diabetes: an observational study. Diabetes Metab 47(5):101212
Blomhoff A, Lie BA, Myhre AG, Kemp EH, Weetman AP, Akselsen HE et al (2004) Polymorphisms in the cytotoxic T lymphocyte antigen-4 gene region confer susceptibility to Addison's disease. J Clin Endocrinol Metab 89(7):3474–3476
Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD et al (2016) Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 101(2):364–389
Brilli L, Calabrò L, Campanile M, Pilli T, Agostinis C, Cerase A et al (2020) Permanent diabetes insipidus in a patient with mesothelioma treated with immunotherapy. Arch Endocrinol Metab 64:483–486
Brunet-Possenti F, Opsomer MA, Gomez L, Ouzaid I, Descamps V (2017) Immune checkpoint inhibitors-related orchitis. Ann Oncol 28:906–907
Castillero F, Castillo-Fernández O, Jiménez-Jiménez G, Fallas-Ramírez J, Peralta-Álvarez MP, Arrieta O (2019) Cancer immunotherapy-associated hypophysitis. Future Oncol 15(27):3159–3169
Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T et al (2016) Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol 186(12):3225–3235
Ceccarini G, Magno S, Gilio D, Pelosini C, Santini F (2021) Autoimmunity in lipodystrophy syndromes. Presse Med 50(3):104073
Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L (2019) Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev 40(1):17–65
Chang CY, Park H, Malone DC, Wang CY, Wilson DL, Yeh YM et al (2020) Immune checkpoint inhibitors and immune-related adverse events in patients with advanced melanoma: a systematic review and network meta-analysis. JAMA Netw Open 3(3):e201611
Chiloiro S, Bianchi A, Giampietro A, Milardi D, De Marinis L, Pontecorvi A (2022) The changing clinical spectrum of endocrine adverse events in cancer immunotherapy. Trends Endocrinol Metab 33(2):87–104
Chmielewska I, Dudzińska M, Szczyrek M, Świrska J, Wojas-Krawczyk K, Zwolak A (2021) Do endocrine adverse events predict longer progression-free survival among patients with non-small-cell lung cancer receiving nivolumab? PLoS One 16(9):e0257484
Cho BC, Yoh K, Perets R, Nagrial A, Spigel DR, Gutierrez M et al (2021) Anti–cytotoxic T-lymphocyte–associated antigen-4 monoclonal antibody quavonlimab in combination with pembrolizumab: safety and efficacy from a phase I study in previously treated extensive-stage small cell lung cancer. Lung Cancer 159:162–170
Cukier P, Santini FC, Scaranti M, Hoff AO (2017) Endocrine side effects of cancer immunotherapy. Endocr Relat Cancer 24(12):T331–TT47
Dadu R, Rodgers TE, Trinh VA, Kemp EH, Cubb TD, Patel S et al (2020) Calcium-sensing receptor autoantibody-mediated hypoparathyroidism associated with immune checkpoint inhibitor therapy: diagnosis and long-term follow-up. J Immunother Cancer 8(1)
Das S, Johnson DB (2019) Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 7(1):306
De Sousa SMC, Sheriff N, Tran CH, Menzies AM, Tsang VHM, Long GV et al (2018) Fall in thyroid stimulating hormone (TSH) may be an early marker of ipilimumab-induced hypophysitis. Pituitary 21(3):274–282
Deligiorgi MV, Sagredou S, Vakkas L, Trafalis DT (2021) The continuum of thyroid disorders related to immune checkpoint inhibitors: still many pending queries. Cancers (Basel) 13(21)
Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S et al (2017) Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab 102(8):2770–2780
El Kawkgi OM, Li D, Kotwal A, Wermers RA (2020) Hypoparathyroidism: an uncommon complication associated With immune checkpoint inhibitor therapy. Mayo Clin Proc Innov Qual Outcomes. 4: © 2020 Mayo Foundation for Medical Education and Research. Elsevier Inc, pp 821–825
Elia G, Ferrari SM, Galdiero MR, Ragusa F, Paparo SR, Ruffilli I et al (2020) New insight in endocrine-related adverse events associated to immune checkpoint blockade. Best Pract Res Clin Endocrinol Metab 34(1):101370
Faje A, Reynolds K, Zubiri L, Lawrence D, Cohen JV, Sullivan RJ et al (2019) Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur J Endocrinol 181(3):211–219
Fröhlich E, Wahl R (2017) Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol 8:521
George J, Bajaj D, Sankaramangalam K, Yoo JW, Joshi NS, Gettinger S et al (2019) Incidence of pancreatitis with the use of immune checkpoint inhibitors (ICI) in advanced cancers: a systematic review and meta-analysis. Pancreatology 19(4):587–594
Gowen MF, Giles KM, Simpson D, Tchack J, Zhou H, Moran U et al (2018) Baseline antibody profiles predict toxicity in melanoma patients treated with immune checkpoint inhibitors. J Transl Med 16(1):82
Grouthier V, Lebrun-Vignes B, Moey M, Johnson DB, Moslehi JJ, Salem JE et al (2020) Immune checkpoint inhibitor-associated primary adrenal insufficiency: WHO VigiBase report analysis. Oncologist 25(8):696–701
Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J et al (2017) Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(suppl_4):iv119–iiv42
Hattersley R, Nana M, Lansdown AJ (2021) Endocrine complications of immunotherapies: a review. Clin Med (Lond) 21(2):e212–ee22
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723
Hommes JW, Verheijden RJ, Suijkerbuijk KPM, Hamann D (2020) Biomarkers of checkpoint inhibitor induced immune-related adverse events-a comprehensive review. Front Oncol 10:585311
Husebye ES, Anderson MS, Kämpe O (2018) Autoimmune Polyendocrine Syndromes. N Engl J Med 378(12):1132–1141
Iglesias P, Sánchez JC, Díez JJ (2021) Isolated ACTH deficiency induced by cancer immunotherapy: a systematic review. Pituitary 24(4):630–643
Inoue H, Park JH, Kiyotani K, Zewde M, Miyashita A, **nin M et al (2016) Intratumoral expression levels of PD-L1, GZMA, and HLA-A along with oligoclonal T cell expansion associate with response to nivolumab in metastatic melanoma. Oncoimmunology 5(9):e1204507
Iravani A, Galligan A, Lasocki A, Wallace R, Weppler A, Au Yeung G et al (2020) FDG PET in the evaluation of immune-related hypophysitis and thyroiditis following combination ipilimumab and nivolumab in advanced melanoma. J Nucl Med 61(supplement 1):482
Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P (2014) Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 6(230):230ra45
Ji HH, Tang XW, Dong Z, Song L, Jia YT (2019) Adverse event profiles of anti-CTLA-4 and anti-PD-1 monoclonal antibodies alone or in combination: analysis of spontaneous reports submitted to FAERS. Clin Drug Investig 39(3):319–330
Joshi MN, Whitelaw BC, Palomar MTP, Wu Y, Carroll PV (2016a) Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: clinical review. Clin Endocrinol 85(3):331–339
Joshi MN, Whitelaw BC, Palomar MT, Wu Y, Carroll PV (2016b) Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: clinical review. Clin Endocrinol 85(3):331–339
Judd J, Zibelman M, Handorf E, O'Neill J, Ramamurthy C, Bentota S et al (2017) Immune-related adverse events as a biomarker in non-melanoma patients treated with programmed cell death 1 inhibitors. Oncologist 22(10):1232–1237
Kanie K, Iguchi G, Bando H, Urai S, Shichi H, Fujita Y et al (2021) Mechanistic insights into immune checkpoint inhibitor-related hypophysitis: a form of paraneoplastic syndrome. Cancer Immunol Immunother 70(12):3669–3677
Kimbara S, Fujiwara Y, Iwama S, Ohashi K, Kuchiba A, Arima H et al (2018) Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci 109(11):3583–3590
Kobayashi T, Iwama S, Sugiyama D, Yasuda Y, Okuji T, Ito M et al (2021) Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. J Immunother Cancer 9(5):e002493
Kotwal A, Haddox C, Block M, Kudva YC (2019) Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care 7(1):e000591
Kotwal A, Kottschade L, Ryder M (2020) PD-L1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid 30(2):177–184
Kotwal A, Rouleau SG, Dasari S, Kottschade L, Ryder M, Kudva YC et al (2021) Immune checkpoint inhibitor-induced hypophysitis: lessons learnt from a large cancer cohort. J Investig Med 70
Kurimoto C, Inaba H, Ariyasu H, Iwakura H, Ueda Y, Uraki S et al (2020) Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci 111(5):1468–1477
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD et al (2019) Five-year survival with combined Nivolumab and Ipilimumab in advanced melanoma. N Engl J Med 381(16):1535–1546
Lasocki A, Iravani A, Galligan A (2021) The imaging of immunotherapy-related hypophysitis and other pituitary lesions in oncology patients. Clin Radiol 76(5):325–332
Lee H, Hodi FS, Giobbie-Hurder A, Ott PA, Buchbinder EI, Haq R et al (2017) Characterization of thyroid disorders in patients receiving immune checkpoint inhibition therapy. Cancer Immunol Res 5(12):1133–1140
Leiter A, Carroll E, Brooks D, Ben Shimol J, Eisenberg E, Wisnivesky JP et al (2021) Characterization of hyperglycemia in patients receiving immune checkpoint inhibitors: beyond autoimmune insulin-dependent diabetes. Diabetes Res Clin Pract 172:108633
Liu J, Zhou H, Zhang Y, Fang W, Yang Y, Huang Y et al (2020) Reporting of immune checkpoint inhibitor therapy–associated diabetes, 2015–2019. Diabetes Care 43(7):e79–e80
Lo Preiato V, Salvagni S, Ricci C, Ardizzoni A, Pagotto U, Pelusi C (2021) Diabetes mellitus induced by immune checkpoint inhibitors: type 1 diabetes variant or new clinical entity? Review of the literature. Rev Endocr Metab Disord 22(2):337–349
Lühder F, Höglund P, Allison JP, Benoist C, Mathis D (1998) Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med 187(3):427–432
Luke JJ, Ott PA (2015) PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget 6(6):3479–3492
Marchand L, Disse E, Dalle S, Reffet S, Vouillarmet J, Fabien N et al (2019a) The multifaceted nature of diabetes mellitus induced by checkpoint inhibitors. Acta Diabetol 56(12):1239–1245
Marchand L, Thivolet A, Dalle S, Chikh K, Reffet S, Vouillarmet J et al (2019b) Diabetes mellitus induced by PD-1 and PD-L1 inhibitors: description of pancreatic endocrine and exocrine phenotype. Acta Diabetol 56(4):441–448
Marin-Acevedo JA, Kimbrough EO, Lou Y (2021) Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol 14(1):45
Michot JM, Ragou P, Carbonnel F, Champiat S, Voisin AL, Mateus C et al (2018) Significance of immune-related lipase increase induced by Antiprogrammed Death-1 or death Ligand-1 antibodies: a brief communication. J Immunother 41(2):84–85
Neppl C, Kaderli RM, Trepp R, Schmitt AM, Berger MD, Wehrli M et al (2018) Histology of Nivolumab-induced thyroiditis. Thyroid 28(12):1727–1728
Nguyen H, Shah K, Waguespack SG, Hu MI, Habra MA, Cabanillas ME et al (2021) Immune checkpoint inhibitor related hypophysitis: diagnostic criteria and recovery patterns. Endocr Relat Cancer 28(7):419–431
O'Malley G, Lee HJ, Parekh S, Galsky MD, Smith CB, Friedlander P et al (2017) Rapid evolution of thyroid dysfunction in patients treated with nivolumab. Endocr Pract 23(10):1223–1231
O'Malley DM, Neffa M, Monk BJ, Melkadze T, Huang M, Kryzhanivska A et al (2021) Dual PD-1 and CTLA-4 checkpoint blockade using Balstilimab and Zalifrelimab combination as second-line treatment for advanced cervical cancer: an open-label phase II study. J Clin Oncol:JCO.21.02067
Özdemir BC (2021) Immune checkpoint inhibitor-related hypogonadism and infertility: a neglected issue in immuno-oncology. J Immunother Cancer 9(2)
Perets R, Bar J, Rasco DW, Ahn MJ, Yoh K, Kim DW et al (2021) Safety and efficacy of quavonlimab, a novel anti-CTLA-4 antibody (MK-1308), in combination with pembrolizumab in first-line advanced non-small-cell lung cancer. Ann Oncol 32(3):395–403
Piranavan P, Li Y, Brown E, Kemp EH, Trivedi N (2019) Immune checkpoint inhibitor-induced hypoparathyroidism associated with calcium-sensing receptor-activating autoantibodies. J Clin Endocrinol Metab 104(2):550–556
Pollack R, Ashash A, Cahn A, Rottenberg Y, Stern H, Dresner-Pollak R (2020) Immune checkpoint inhibitor-induced thyroid dysfunction is associated with higher body mass index. J Clin Endocrinol Metab 105(10)
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378(2):158–168
Quach HT, Robbins CJ, Balko JM, Chiu CY, Miller S, Wilson MR et al (2019) Severe Epididymo-Orchitis and encephalitis complicating anti-PD-1 therapy. Oncologist 24(7):872–876
Quandt Z, Young A, Anderson M (2020) Immune checkpoint inhibitor diabetes mellitus: a novel form of autoimmune diabetes. Clin Exp Immunol 200(2):131–140
Quinn M, Joshi M, Carroll PV (2021) Endocrine effects of immunotherapy for cancer. Medicine 49(9):554–557
Raschi E, Mazzarella A, Antonazzo IC, Bendinelli N, Forcesi E, Tuccori M et al (2019) Toxicities with immune checkpoint inhibitors: emerging priorities from disproportionality analysis of the FDA adverse event reporting system. Target Oncol 14(2):205–221
Rosenblum MD, Remedios KA, Abbas AK (2015) Mechanisms of human autoimmunity. J Clin Invest 125(6):2228–2233
Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA (2014) Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer 21(2):371–381
Salinas C, Renner A, Rojas C, Samtani S, Burotto M (2020) Primary adrenal insufficiency during immune checkpoint inhibitor treatment: case reports and review of the literature. Case Rep Oncol 13(2):621–626
Scovell JM, Benz K, Samarska I, Kohn TP, Hooper JE, Matoso A et al (2020) Association of Impaired Spermatogenesis with the use of immune checkpoint inhibitors in patients with metastatic melanoma. JAMA Oncol 6(8):1297–1299
Seidel JA, Otsuka A, Kabashima K (2018) Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 8:86
Shi Y, Shen M, Zheng X, Chen Y, Zhao R, Gu Y et al (2020) ICPis-induced autoimmune Polyendocrine syndrome type 2: a review of the literature and a protocol for optimal management. J Clin Endocrinol Metab 105(12)
Shulgin B, Kosinsky Y, Omelchenko A, Chu L, Mugundu G, Aksenov S et al (2020) Dose dependence of treatment-related adverse events for immune checkpoint inhibitor therapies: a model-based meta-analysis. Onco Targets Ther 9(1):1748982
Siddiqui MS, Lai ZM, Spain L, Greener V, Turajlic S, Larkin J et al (2021) Predicting development of ipilimumab-induced hypophysitis: utility of T4 and TSH index but not TSH. J Endocrinol Investig 44(1):195–203
Thapi S, Leiter A, Galsky M, Gallagher EJ (2019) Recovery from secondary adrenal insufficiency in a patient with immune checkpoint inhibitor therapy induced hypophysitis. J Immunother Cancer 7(1):248
Trinh B, Sanchez GO, Herzig P, Läubli H (2019a) Inflammation-induced hypoparathyroidism triggered by combination immune checkpoint blockade for melanoma. J Immunother Cancer 7(1):52
Trinh B, Donath MY, Läubli H (2019b) Successful treatment of immune checkpoint inhibitor-induced diabetes with infliximab. Diabetes Care 42(9):e153–e1e4
Tsoli M, Kaltsas G, Angelousi A, Alexandraki K, Randeva H, Kassi E (2020) Managing Ipilimumab-induced Hypophysitis: challenges and current therapeutic strategies. Cancer Manag Res 12:9551–9561
Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G et al (2003) Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423(6939):506–511
Venetsanaki V, Boutis A, Chrisoulidou A, Papakotoulas P (2019) Diabetes mellitus secondary to treatment with immune checkpoint inhibitors. Curr Oncol 26(1):e111–e1e4
Weber JS, Postow M, Lao CD, Schadendorf D (2016) Management of Adverse Events Following Treatment with Anti-Programmed Death-1 agents. Oncologist 21(10):1230–1240
Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J et al (2017) Safety profile of Nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 35(7):785–792
Wu L, Tsang VHM, Sasson SC, Menzies AM, Carlino MS, Brown DA et al (2021) Unravelling checkpoint inhibitor associated autoimmune diabetes: from bench to bedside. Front Endocrinol (Lausanne) 12:764138
**a L, Peng J, Lou G, Pan M, Zhou Q, Hu W et al (2022) Antitumor activity and safety of camrelizumab plus famitinib in patients with platinum-resistant recurrent ovarian cancer: results from an open-label, multicenter phase 2 basket study. J Immunother Cancer 10(1)
Yamamoto N, Tsurutani Y, Katsuragawa S, Kubo H, Sunouchi T, Hirose R et al (2019) A patient with Nivolumab-related fulminant type 1 diabetes mellitus whose serum C-peptide level was preserved at the initial detection of Hyperglycemia. Internal Med (Tokyo, Japan) 58(19):2825–2830
Yamauchi I, Sakane Y, Fukuda Y, Fujii T, Taura D, Hirata M et al (2017) Clinical features of Nivolumab-induced thyroiditis: a case series study. Thyroid 27(7):894–901
Yano S, Ashida K, Sakamoto R, Sakaguchi C, Ogata M, Maruyama K et al (2020) Human leucocyte antigen DR15, a possible predictive marker for immune checkpoint inhibitor-induced secondary adrenal insufficiency. Eur J Cancer 130:198–203
Yoneda S, Imagawa A, Hosokawa Y, Baden MY, Kimura T, Uno S et al (2019) T-lymphocyte infiltration to islets in the pancreas of a patient who developed type 1 diabetes after administration of immune checkpoint inhibitors. Diabetes Care 42(7):e116–e1e8
Zhan L, Feng HF, Liu HQ, Guo LT, Chen C, Yao XL et al (2021) Immune checkpoint inhibitors-related thyroid dysfunction: epidemiology, clinical presentation, possible pathogenesis, and management. Front Endocrinol (Lausanne). 12:649863
Zhang AL, Wang F, Chang LS, McDonnell ME, Min L (2021) Coexistence of immune checkpoint inhibitor-induced autoimmune diabetes and pancreatitis. Front Endocrinol (Lausanne). 12:620522
Zhao Z, Wang X, Bao XQ, Ning J, Shang M, Zhang D (2021) Autoimmune polyendocrine syndrome induced by immune checkpoint inhibitors: a systematic review. Cancer Immunol Immunother 70(6):1527–1540
Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F (2020) Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med 18(1):87
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this entry
Cite this entry
Quinn, M., Carroll, P.V., Joshi, M.N. (2022). Endocrine Toxicities Related to Immunotherapy. In: Rezaei, N. (eds) Handbook of Cancer and Immunology. Springer, Cham. https://doi.org/10.1007/978-3-030-80962-1_348-1
Download citation
DOI: https://doi.org/10.1007/978-3-030-80962-1_348-1
Received:
Accepted:
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80962-1
Online ISBN: 978-3-030-80962-1
eBook Packages: Springer Reference Biomedicine and Life SciencesReference Module Biomedical and Life Sciences