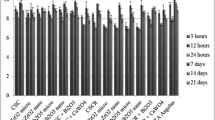
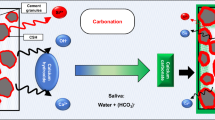
Abstract
Hydraulic calcium silicate cements have been developed to be used in moist clinical environments. When they react with water, their hydration results in the formation of calcium hydroxide which is necessary for a number of clinical uses. The first clinically available hydraulic calcium silicate cement—mineral trioxide aggregate—exhibited a number of shortcomings. Further materials have been developed aimed at addressing the shortcomings of the original formulation. Biodentine is a hydraulic calcium silicate cement, which has been optimized to exhibit an ordered microstructure and aiming at improved physical, chemical and biological properties.
Biodentine is primarily composed of laboratory-grade tricalcium silicate cement, includes zirconium oxide as radiopacifier and calcium carbonate, calcium chloride and a hydro-soluble polymer as reaction modifiers. It is classified as a Type 4 cement. Its specific composition leads to the high early calcium ion release, short setting time, low porosity, improved mechanical properties and improved material handling. This makes Biodentine ideal for clinical procedures involving the vital pulp.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Duncan HF, Galler KM, Tomson PL, Simon S, El-Karim I, et al. European Society of Endodontology position statement: management of deep caries and the exposed pulp. Int Endod J. 2019;52:923–34.
Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-cap** agents in human teeth: a preliminary report. Int Endod J. 2003;36:225–31.
Nair PNR, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental cap** with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. 2008;41:128–50.
Cho SY, Seo DG, Lee SJ, Lee J, Lee SJ, Jung IY. Prognostic factors for clinical outcomes according to time after direct pulp cap**. J Endod. 2013;39:327–31.
Hilton TJ, Ferracane JL, Mancl L, Northwest Practice-Based Research Collaborative in Evidence-Based Dentistry (NWP). Comparison of CaOH with MTA for direct pulp cap**: a PBRN randomized clinical trial. J Dent Res. 2013;92:16S–22S.
Mente J, Hufnagel S, Leo M, et al. Treatment outcome of mineral trioxide aggregate or calcium hydroxide direct pulp cap**: long-term results. J Endod. 2014;40:1746–51.
Kundzina R, Stangvaltaite L, Eriksen HM, Kerosuo E. Cap** carious exposures in adults: a randomized controlled trial investigating mineral trioxide aggregate versus calcium hydroxide. Int Endod J. 2017;50:924–32.
Witte DR. The filling of a root canal with Portland cement. German quarterly for dentistry. J Cent Assoc German Dent. 1878;18:153–4.
Schlenker M. Fuellen der Wurzelkanaele mit Portland-Cement nach Dr Witte [Classification of clinically available hydraulic calcium silicate cements]. Deutsche Vrtljschr F Zahnh. 1880;20:277–83.
Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993;19(11):541–4.
Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993;19(12):591–5.
Torabinejad M, White JD. Tooth filling material and method of use. Patent number: 5415547, 1993.
Torabinejad M, White JD. Tooth filling material and method of use. Patent number: 5769638, 1995.
Camilleri J. Classification of hydraulic cements used in dentistry. Front Dent Med. 2020;1:9. https://doi.org/10.3389/fdmed.2020.00009.
Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Pitt Ford TR. The constitution of mineral trioxide aggregate. Dent Mater. 2005;21:297–303.
Camilleri J, Sorrentino F, Damidot D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent Mater. 2013;29(5):580–93.
Camilleri J, Kralj P, Veber M, Sinagra E. Characterization and analyses of acid-extractable and leached trace elements in dental cements. Int Endod J. 2012;45(8):737–43.
Camilleri J. Characterization and hydration kinetics of tricalcium silicate cement for use as a dental biomaterial. Dent Mater. 2011;27(8):836–44.
Chang SW, Shon WJ, Lee W, Kum KY, Baek SH, Bae KS. Analysis of heavy metal contents in gray and white MTA and 2 kinds of Portland cement: a preliminary study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(4):642–6.
Schembri M, Peplow G, Camilleri J. Analyses of heavy metals in mineral trioxide aggregate and Portland cement. J Endod. 2010;36(7):1210–5.
Monteiro Bramante C, Demarchi AC, de Moraes IG, Bernadineli N, Garcia RB, Spångberg LS, Duarte MA. Presence of arsenic in different types of MTA and white and gray Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(6):909–13.
De-Deus G, de Souza MC, Sergio Fidel RA, Fidel SR, de Campos RC, Luna AS. Negligible expression of arsenic in some commercially available brands of Portland cement and mineral trioxide aggregate. J Endod. 2009;35(6):887–90.
Kum KY, Zhu Q, Safavi K, Gu Y, Bae KS, Chang SW. Analysis of six heavy metals in Ortho mineral trioxide aggregate and ProRoot mineral trioxide aggregate by inductively coupled plasma-optical emission spectrometry. Aust Endod J. 2013;39(3):126–30.
Duarte MA, De Oliveira Demarchi AC, Yamashita JC, Kuga MC, De Campos Fraga S. Arsenic release provided by MTA and Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(5):648–50.
Demirkaya K, Can Demirdöğen B, Öncel Torun Z, Erdem O, Çetinkaya S, Akay C. In vivo evaluation of the effects of hydraulic calcium silicate dental cements on plasma and liver aluminium levels in rats. Eur J Oral Sci. 2016;124(1):75–81.
Demirkaya K, Demirdöğen BC, Torun ZÖ, Erdem O, Çırak E, Tunca YM. Brain aluminium accumulation and oxidative stress in the presence of calcium silicate dental cements. Hum Exp Toxicol. 2017;36(10):1071–80. https://doi.org/10.1177/0960327116679713. Epub 2016 Nov 27.
Simsek N, Bulut ET, Ahmetoğlu F, Alan H. Determination of trace elements in rat organs implanted with endodontic repair materials by ICP-MS. J Mater Sci Mater Med. 2016;27(3):46.
Garcia LDFR, Huck C, Magalhães FAC, Souza PPC, Souza Costa CA. Systemic effect of mineral aggregate-based cements: histopathological analysis in rats. J Appl Oral Sci. 2017;25(6):620–30. https://doi.org/10.1590/1678-7757-2016-0634.
Taylor HFW. Cement chemistry. London: Thomas Telford; 1997. p. 113–225.
Odler I. Hydration, setting and hardening of Portland cement. In: Hewlett PC, editor. Lea’s chemistry of cement and concrete. London: Arnold; 1998. p. 241–84.
Moir GK. Cements. In: Newman J, Choo BS, editors. Advanced concrete technology; constituent materials. Elsevier Butterworth Heinemann: Oxford; 2003. p. 3–45.
Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007;40(6):462–70.
Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008;41(5):408–17.
Arias-Moliz MT, Farrugia C, Lung CYK, Schembri Wismayer P, Camilleri J. Antimicrobial and biological activity of leachate from light curable pulp cap** materials. J Dent. 2017;64:45–51.
Camilleri J, Laurent P, About I. Hydration of Biodentine, Theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp cap** in entire tooth cultures. J Endod. 2014;40(11):1846–54.
Kaup M, Schäfer E, Dammaschke T. An in vitro study of different material properties of Biodentine™ compared to ProRoot MTA. Head Face Med. 2015;11:16.
Grech L, Mallia B, Camilleri J. Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent Mater. 2013;29(2):e20–8.
Grech L, Mallia B, Camilleri J. Characterization of set intermediate restorative material, Biodentine, bioaggregate and a prototype calcium silicate cement for use as root-end filling materials. Int Endod J. 2013;46(7):632–41.
Camilleri J, Grech L, Galea K, Keir D, Fenech M, Formosa L, Damidot D, Mallia B. Porosity and root dentine to material interface assessment of calcium silicate-based root-end filling materials. Clin Oral Investig. 2014;18(5):1437–46.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Camilleri, J. (2022). BiodentineTM Microstructure and Composition. In: About, I. (eds) Biodentine™. Springer, Cham. https://doi.org/10.1007/978-3-030-80932-4_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-80932-4_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80931-7
Online ISBN: 978-3-030-80932-4
eBook Packages: MedicineMedicine (R0)