Abstract
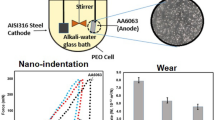
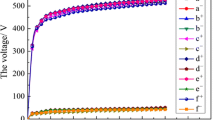
The chapter is devoted to the study of morphology, topography, and fractality of heterooxide coatings formed on common structural materials—mild steel and aluminum alloys. The aim of the work was to investigate the regularities of changes in the characteristics of surface layers depending on the method of electrosynthesis and their influence on the properties of the obtained functional materials. Rational modes of electrolysis for the formation of coatings enriched with the target dopant component are substantiated. In particular, it is shown that varying the type of polarization (direct or pulsed current) makes it possible to control the molybdenum content in the Fe–Co–MoOx coatings within the range of 18–38 at. %. The synthesis of heterooxide coatings Al2O3 · CoOx (MnOy) with a content of cobalt up to 24 at. % and manganese up to 36 at. % should be carried out by plasma electrolytic oxidation in the mode of “decreasing power” with the current density of 3–20 A/dm2. According to the results of research, it is shown that the indicators of roughness and fractality are influenced by the method of synthesis of electrochemical coating and the composition of the processed material. The fractal dimension of Fe–Co–MoOx coatings formed by direct current varies in the range of 2.05–2.64 and in the range of 2.47–2.69 for coatings formed in the pulse mode. Moreover, the values of D depend on the scanning area of the selected area. Thus, local surface alignment is observed during the transition to nanoscale relief. For oxide coatings on aluminum, an increase in fractality during the incorporation of cobalt and manganese into the surface layers has been established.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Tsyntsaru N, Cesiulis H, Donten M, Sort J, Pellicer E, Podlaha-Murphy EJ (2012) Modern trends in tungsten alloys electrodeposition with iron group metals. Surf Eng Appl Electrochem 48(6):491–520
Paulo N, Casciano S, Benevides RL, Lima-Neto P, Adriana N (2014) Corrosion resistance of electrodeposited Ni-Mo-W coatings. Correia Int J Electrochem Sci 9:4413–4428
Kublanovskii VS, Yapontseva YuS, Troshchenkov YuN et al (2010) Corrosion and magnetic propetries of electrolytic Co-Mo alloys. J Appl Electrochem 8(3):440–444
Rudnev VS, Lukiyanchuk IV, Vasilyeva MS, Medkov MA, Adigamova MV, Sergienko VI (2016) Aluminum- and titanium-supported plasma electrolytic multicomponent coatings with magnetic, catalytic, biocide or biocompatible properties. Surf Coat Tech 307(C):1219–1235. https://doi.org/10.1016/j.surfcoat.2016.07.060
Tsyntsaru N, Bobanova J, Ye X, Cesiulis H, Dikusar A, Prosycevas I, Celis J-P (2009) Iron-Tungsten alloys electrodeposited under direct current from citrate–ammonia plating bath. Surf Coat Technol 203:3136–3141
Su F-H, Huang P (2012) Microstructure and tribological property of nanocrystalline Co-W alloy coating produced by dual-pulse electrodeposition. Mater Chem Phys 134:350–359
Martin J, Leone P, Nomine A, Veys-Renaux D, Henrion G, Belmonte T (2015) Influence of electrolyte ageing on the plasma electrolytic oxidation of aluminium. Surf Coat Technol 269:36–46
Mohedano M, Lu X, Matykina E, Blawert C, Arrabal R, Zheludkevich ML (2017) plasma electrolytic oxidation (PEO) of metals and alloys. In: Book: reference module in chemistry, molecular sciences and chemical engineering, pp 432–437. https://doi.org/10.1016/B978-0-12-409547-2.13398-0
Jiang BL, Wang YM (2010) Plasma electrolytic oxidation treatment of aluminium and titanium alloys. Surf Eng Light Alloys 110–154
Capel H, Shipway PH, Harris SJ (2003) Wear 255:917. https://doi.org/10.1016/S0043-1648(03)00241-2
Yapontseva YS, Dikusar AI, Kyblanovskii VS (2014) Study of the composition, corrosion, and catalytic properties of Co-W alloys electrodeposited from a citrate pyrophosphate electrolyte. Surf Eng Appl Electrochem 50:330. https://doi.org/10.3103/S1068375514040139
Donten M, Cesiulis H, Stojek Z (2000) Electrodeposition and properties of Ni-W, Fe-W and Ni-Fe-W amorphous alloys. A comparative study. Electrochim Acta 45(20):3389–3396
Safizadeh F, Sorour N, Ghali E, Houlachi G (2017) Study of the hydrogen evolution reaction on Fe-Mo-P coatings as cathodes for chlorate production. Intern J Hydrogen Energy 42(8):5455–5463
Gomez E, Pellicer E, Valles E (2001) Electrodeposited cobalt_molybdenum magnetic materials. J Electroanal Chem 517:109–116
Gomez E, Pellicer E, Alcobe X, Valles E (2004) Properties of Co-Mo coatings obtained by electrodeposition at pH 6.6. J Solid State Eletrochem 8:497–504
Gong J, Riemer S, Morrone A, Venkatasamy V, Kautzky M, Tabakovic I (2012) Composition gradients and magnetic properties of 5–100 nm thin CoNiFe films obtained by electrodeposition. J Electrochem Soc 159(7):D447–D454
Tabakovic I, Gong J, Riemer S, Kautzky M (2015) Influence of surface roughness and current efficiency on composition gradients of thin NiFe films obtained by electrodeposition electrochemical/electroless deposition. J Electrochem Soc 162:D102–D108
Frederix P, Linford MR (2017) Surface analysis by atomic force microscopy (AFM): its various modes, capabilities, and comparison to and integration with other techniques. Vacuum technology & coating
Gan Sh, Zhou Q, Xu X, Hong Y, Liu Y, Fu Sh (2007) Study on the surface roughness of substrate with multi-fractal spectrum. Microelectron Eng 84:1806–1809. https://doi.org/10.1016/j.mee.2007.01.273
Stout K, Blunt L (2000) Three-dimensional surface topography, 2nd edition (Ultra precision technology). Penton Press, London, p 285
Kotowski P (2006) Fractal dimension of metallic fracture surface. Int J Fract 141:269–286. https://doi.org/10.1007/s10704-006-8264-x
Menchaca-Campos EC, Villalba-Enciso ER, Juarez-Nuñez V, Flores-Dominguez M, Mayorga-Cruz D, Tapia RG, Uruchurtu-Chavarín J (2020) Fractal dimension analysis of aluminum corrosion roughness by electrochemical and optical methods. Euro J Eng Res Sci 5(3)
Xu S, Weng Y (2006) A new approach to estimate fractal dimensions of corrosion images. Pattern Recognit Lett 27(16):1942–1947
Garcia R, Perez R (2002) Dynamic atomic force microscopy methods. Surf Sci Rep 47:197–301
Yermolenko IYu, Ved MV, Sakhnenko ND (2019) The kinetics peculiarities and the electrolysis regime effect on the morphology and phase composition of Fe-Co-W(Mo) coatings. Chapter 28: In: Fesenko O, Yatsenko L (eds) Nanocomposites, nanostructures, and their applications. NANO 2018. Springer proceedings in physics, vol 221. Springer, Cham, Switzerland AG 2019, pp 403–423. https://doi.org/10.1007/978-3-030-17759-1_28
Ved MV, Sakhnenko ND, Yermolenko IYu, Nenastina TA (2018) Nanostructured functional coatings of iron family metals with refractory elements. In: Fesenko O, Yatsenko L (eds) Chapter 1: in the book nanochemistry, biotechnology, nanomaterials, and their applications, Springer proceedings in physics 214. Springer International Publishing AG, part of Springer Nature 2018, pp 3–34. https://doi.org/10.1007/978-3-319-92567-7_1
Yermolenko IY, Ved MV, Sakhnenko ND, Sachanova YI (2017) Composition, morphology, and topography of galvanic coatings Fe-Co-W and Fe-Co-Mo. Nanoscale Res Lett 12(1):352. https://doi.org/10.1186/s11671-017-2128-3
Yermolenko IYu, Ved MV, Sakhnenko ND, Shipkova IG, Zyubanova SI (2019) Nanostructured magnetic films based on iron with refractory metals. J Mag Magn Mater 475:115–120. https://doi.org/10.1016/j.jmmm.2018.11.104
Karakurkchi AV, Sakhnenko ND, Ved MV, Luhovskyi IS, Drobakha HA, Mayba MV (2019) Features of plasma electrolytic formation of manganese- and cobalt-containing composites on aluminum alloys. Adv Mater Sci Eng 6381291:13. https://doi.org/10.1155/2019/6381291
Sakhnenko M, Ved MV, Karakurkchi A (2017) Nanoscale oxide PEO coatings forming from diphosphate electrolytes. Nanophys Nanomater Interface Stud Appl NANO 2016 195:507–531. https://doi.org/10.1007/978-3-319-56422-7_38
Karakurkchi AV, Sakhnenko MD, Ved’ MV, Tulenko MV, Dzheniuk AV (2020) Analysis of technological approaches to electrochemical surface treatment of aluminum alloys. Eastern-Euro J Enterp Technol Mater Sci 3(12(105)):44−55. https://doi.org/10.15587/1729-4061.2020.206014
Parsadanov IV, Sakhnenko ND, Ved MV, Rykova IV, Khyzhniak VA, Karakurkchi AV, Gorokhivskiy AS (2017) Increasing the efficiency of intra-cylinder catalysis in diesel engines. Voprosy Khimii i Khimicheskoi Tekhnologii 6:75–81
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Yermolenko, I.Y., Karakurkchi, H.V., Ved, M.V., Sakhnenko, N.D. (2021). The Investigation of Morphology, Topography, and Surface Fractality of Heterooxide Composite Coatings. In: Fesenko, O., Yatsenko, L. (eds) Nanomaterials and Nanocomposites, Nanostructure Surfaces, and Their Applications . NANO 2020. Springer Proceedings in Physics, vol 263. Springer, Cham. https://doi.org/10.1007/978-3-030-74741-1_31
Download citation
DOI: https://doi.org/10.1007/978-3-030-74741-1_31
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-74740-4
Online ISBN: 978-3-030-74741-1
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)