Abstract
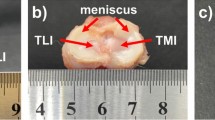
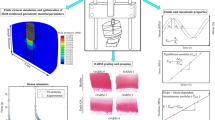
The knowledge of site-specific properties of articular cartilage of a knee joint may be important for understanding the onset of cartilage degeneration in the knee. Few earlier studies have focused on the rate-dependent poromechanical response of knee joints to site-specific material properties across the joint. The objective of the present study was to develop a methodology to implement the in-situ cartilage mechanical properties in an anatomically accurate computational model of the porcine knee joint. Fresh porcine knee joints were used to reconstruct the knee geometry using magnetic resonance imaging. An automated indentation test was used to determine the site-specific cartilage properties. The variations of the recorded reaction forces over different sites were not solely due to nonuniform cartilage thickness. The nonfibrillar matrix and fibrillar network of the tibial cartilage had higher stiffness compared to that of the femoral cartilage as determined in the data fitting procedure. Considering the site-specific properties in finite element simulations, the force-compression relationship of the joint was determined by both compression-magnitude and compression-rate. The preliminary results indicated that a realistic implementation of site-specific tissue properties may be necessary for understanding the load distribution in the joint. The methodology will be further refined and tested.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Sophia-Fox, A.J., Bedi, A., Rodeo, S.A.: The basic science of articular cartilage: structure, composition, and function. Sport. Health Multidiscip. Approach 1, 461–468 (2009). https://doi.org/10.1177/1941738109350438
Troken, A.J., Mao, J.J., Marion, N.W., Wan, L.Q., Mow, V.C.: Cartilage and meniscus, properties of. In: Encyclopedia of Medical Devices and Instrumentation. Wiley, Hoboken (2006)
Wang, Y., Ding, C., Wluka, A.E., Davis, S., Ebeling, P.R., Jones, G., Cicuttini, F.M.: Factors affecting progression of knee cartilage defects in normal subjects over 2 years. Rheumatology 45, 79–84 (2006). https://doi.org/10.1093/rheumatology/kei108
Korhonen, R.K., Julkunen, P., Li, L.P., van Donkelaar, C.C.: Computational models of articular cartilage. Comput. Math. Methods Med. 2013, 254507 (2013). https://doi.org/10.1155/2013/254507
Huber, M., Trattnig, S., Lintner, F.: Anatomy, biochemistry, and physiology of articular cartilage. Invest. Radiol. 35, 573–580 (2000)
Bhosale, A.M., Richardson, J.B.: Articular cartilage: structure, injuries and review of management. Br. Med. Bull. 87, 77–95 (2008). https://doi.org/10.1093/bmb/ldn025
Mow, V.C., Huiskes, R.: Basic Orthopaedic Biomechanics & Mechano-biology. Lippincott Williams & Wilkins, Philadelphia (2005)
Taylor, Z.A., Miller, K.: Constitutive modeling of cartilaginous tissues: a review. J. Appl. Biomech. 22, 212–229 (2006)
Li, L.P., Soulhat, J., Buschmann, M.D., Shirazi-Adl, A.: Nonlinear analysis of cartilage in unconfined ramp compression using a fibril reinforced poroelastic model. Clin. Biomech. 14, 673–682 (1999). https://doi.org/10.1016/S0268-0033(99)00013-3
Gao, L.L., Zhang, C.Q., Gao, H., Liu, Z.D., **ao, P.P.: Depth and rate dependent mechanical behaviors for articular cartilage: experiments and theoretical predictions. Mater. Sci. Eng. C 38, 244–251 (2014). https://doi.org/10.1016/J.MSEC.2014.02.009
Oloyede, A., Broom, N.: Stress-sharing between the fluid and solid components of articular cartilage under varying rates of compression. Connect. Tissue Res. 30, 127–141 (1993)
Li, L.P., Herzog, W.: Strain-rate dependence of cartilage stiffness in unconfined compression: the role of fibril reinforcement versus tissue volume change in fluid pressurization. J. Biomech. 37, 375–382 (2004)
Boschetti, F., Pennati, G., Gervaso, F., Peretti, G.M., Dubini, G.: Biomechanical properties of human articular cartilage under compressive loads. Biorheology 41, 159–166 (2004)
DiSilvestro, M.R., Suh, J.K.F.: A cross-validation of the biphasic poroviscoelastic model of articular cartilage in unconfined compression, indentation, and confined compression. J. Biomech. 34, 519–525 (2001). https://doi.org/10.1016/S0021-9290(00)00224-4
Korhonen, R.K., Laasanen, M.S., Töyräs, J., Rieppo, J., Hirvonen, J., Helminen, H.J., Jurvelin, J.S.: Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J. Biomech. 35, 903–909 (2002). https://doi.org/10.1016/S0021-9290(02)00052-0
Mow, V.C., Gibbs, M.C., Lai, W.M., Zhu, W.B., Athanasiou, K.A.: Biphasic indentation of articular cartilage—II. A numerical algorithm and an experimental study. J. Biomech. 22, 853–861 (1989). https://doi.org/10.1016/0021-9290(89)90069-9
Chen, X., Zimmerman, B.K., Lu, X.L.: Determine the equilibrium mechanical properties of articular cartilage from the short-term indentation response. J. Biomech. 48, 176–180 (2015). https://doi.org/10.1016/J.JBIOMECH.2014.10.036
Cao, L., Youn, I., Guilak, F., Setton, L.A.: Compressive properties of mouse articular cartilage determined in a novel micro-indentation test method and biphasic finite element model. J. Biomech. Eng. 128, 766 (2006). https://doi.org/10.1115/1.2246237
Lu, X.L., Mow, V.C., Guo, X.E.: Proteoglycans and mechanical behavior of condylar cartilage. J. Dent. Res. 88, 244–248 (2009). https://doi.org/10.1177/0022034508330432
Hosoda, N., Sakai, N., Sawae, Y., Murakami, T.: Finite element analyses of articular cartilage models considering depth-dependent elastic modulus and collagen fiber network. J. Biomech. Sci. Eng. 5, 437–448 (2010). https://doi.org/10.1299/jbse.5.437
Klika, V., Gaffney, E.A., Chen, Y.C., Brown, C.P.: An overview of multiphase cartilage mechanical modelling and its role in understanding function and pathology. J. Mech. Behav. Biomed. Mater. 62, 139–157 (2016). https://doi.org/10.1016/J.JMBBM.2016.04.032
DiSilvestro, M.R., Zhu, Q., Wong, M., Jurvelin, J.S., Suh, J.K.F.: Biphasic poroviscoelastic simulation of the unconfined compression of articular cartilage: I—simultaneous prediction of reaction force and lateral displacement. J. Biomech. Eng. 123, 191 (2001). https://doi.org/10.1115/1.1351890
Richard, F., Villars, M., Thibaud, S.: Viscoelastic modeling and quantitative experimental characterization of normal and osteoarthritic human articular cartilage using indentation. J. Mech. Behav. Biomed. Mater. 24, 41–52 (2013). https://doi.org/10.1016/J.JMBBM.2013.04.012
Mäkelä, J.T.A., Han, S.K., Herzog, W., Korhonen, R.K.: Very early osteoarthritis changes sensitively fluid flow properties of articular cartilage. J. Biomech. 48, 3369–3376 (2015). https://doi.org/10.1016/J.JBIOMECH.2015.06.010
Bendjaballah, M., Shirazi-Adl, A., Zukor, D.: Biomechanics of the human knee joint in compression: reconstruction, mesh generation and finite element analysis. Knee 2, 69–79 (1995)
Li, G., Gil, J., Kanamori, A., Woo, S.L.: A validated three-dimensional computational model of a human knee joint. J. Biomech. Eng. 121, 657–662 (1999). https://doi.org/10.1115/1.2800871
Gu, K.B., Li, L.P.: A human knee joint model considering fluid pressure and fiber orientation in cartilages and menisci. Med. Eng. Phys. 33, 497–503 (2011)
Below, S., Arnoczky, S.P., Dodds, J., Kooima, C., Walter, N.: The split-line pattern of the distal femur: a consideration in the orientation of autologous cartilage grafts. Arthrosc. J. Arthrosc. Relat. Surg. 18, 613–617 (2002). https://doi.org/10.1053/JARS.2002.29877
Shim, V.B., Besier, T.F., Lloyd, D.G., Mithraratne, K., Fernandez, J.F.: The influence and biomechanical role of cartilage split line pattern on tibiofemoral cartilage stress distribution during the stance phase of gait. Biomech. Model. Mechanobiol. 15, 195–204 (2016). https://doi.org/10.1007/s10237-015-0668-y
Kiapour, A., Kiapour, A.M., Kaul, V., Quatman, C.E., Wordeman, S.C., Hewett, T.E., Demetropoulos, C.K., Goel, V.K.: Finite element model of the knee for investigation of injury mechanisms: development and validation. J. Biomech. Eng. 136, 011002 (2013). https://doi.org/10.1115/1.4025692
Mootanah, R., Imhauser, C.W., Reisse, F., Carpanen, D., Walker, R.W., Koff, M.F., Lenhoff, M.W., Rozbruch, S.R., Fragomen, A.T., Dewan, Z., Kirane, Y.M., Cheah, K., Dowell, J.K., Hillstrom, H.J.: Development and validation of a computational model of the knee joint for the evaluation of surgical treatments for osteoarthritis. Comput. Methods Biomech. Biomed. Eng. 17, 1502–1517 (2014). https://doi.org/10.1080/10255842.2014.899588
Mononen, M.E., Tanska, P., Isaksson, H., Korhonen, R.K.: A novel method to simulate the progression of collagen degeneration of cartilage in the knee: data from the osteoarthritis initiative. Sci. Rep. 6, 21415 (2016). https://doi.org/10.1038/srep21415
Mononen, M.E., Tanska, P., Isaksson, H., Korhonen, R.K.: New algorithm for simulation of proteoglycan loss and collagen degeneration in the knee joint: data from the osteoarthritis initiative. J. Orthop. Res. 36, 1673–1683 (2018). https://doi.org/10.1002/jor.23811
Erdemir, A., Besier, T.F., Halloran, J.P., Imhauser, C.W., Laz, P.J., Morrison, T.M., Shelburne, K.B.: Deciphering the “art” in modeling and simulation of the knee joint: overall strategy. J. Biomech. Eng. 141, 071002 (2019). https://doi.org/10.1115/1.4043346
Proffen, B.L., McElfresh, M., Fleming, B.C., Murray, M.M.: A comparative anatomical study of the human knee and six animal species. Knee 19, 493–499 (2012). https://doi.org/10.1016/J.KNEE.2011.07.005
Lavoie, J.F., Sim, S., Quenneville, E., Garon, M., Moreau, A., Buschmann, M.D., Aubin, C.E.: Map** articular cartilage biomechanical properties of normal and osteoarthritic mice using indentation. In: Osteoarthritis Research Society International, Seattle, WA, USA (2015)
Sim, S., Matuska, A., Garon, M., Quenneville, E., McFetridge, P., Buschmann, M.D.: Cartilage stiffness and thickness distributions revealed by an automated indentation technique in the temporomandibular joint. In: TMJ Bioengineering Conference, Barcelona, Spain (2016)
Moshtagh, P.R., Pouran, B., Korthagen, N.M., Zadpoor, A.A., Weinans, H.: Guidelines for an optimized indentation protocol for measurement of cartilage stiffness: the effects of spatial variation and indentation parameters. J. Biomech. 49, 3602–3607 (2016). https://doi.org/10.1016/J.JBIOMECH.2016.09.020
Rieppo, J., Hyttinen, M.M., Halmesmaki, E., Ruotsalainen, H., Vasara, A., Kiviranta, I., Jurvelin, J.S., Helminen, H.J.: Changes in spatial collagen content and collagen network architecture in porcine articular cartilage during growth and maturation. Osteoarthr. Cartil. 17, 448–455 (2009). https://doi.org/10.1016/J.JOCA.2008.09.004
Kazemi, M., Li, L.P.: A viscoelastic poromechanical model of the knee joint in large compression. Med. Eng. Phys. 36, 998–1006 (2014). https://doi.org/10.1016/J.MEDENGPHY.2014.04.004
Donahue, T.L., Hull, M.L., Rashid, M.M., Jacobs, C.R.: A finite element model of the human knee joint for the study of tibio-femoral contact. J. Biomech. Eng. 124, 273 (2002). https://doi.org/10.1115/1.1470171
Teeple, E., Fleming, B.C., Mechrefe, A.P., Crisco, J.J., Brady, M.F., Jay, G.D.: Frictional properties of Hartley guinea pig knees with and without proteolytic disruption of the articular surfaces. Osteoarthr. Cartil. 15, 309–315 (2007). https://doi.org/10.1016/J.JOCA.2006.08.011
Lu, X.L., Miller, C., Quo, X.E., Van Mow, C.: A new correspondence principle for triphasic materials: determination of fixed charge density and porosity of articular cartilage by indentation. ASME-BED, Vail, Colorado (2005)
Pal, S.: Mechanical properties of biological materials. In: Design of Artificial Human Joints & Organs, pp. 23–40. Springer US, Boston (2014)
Rodriguez, M.L., Li, L.P.: Compression-rate-dependent nonlinear mechanics of normal and impaired porcine knee joints. BMC Musculoskelet. Disord. 18, 1–10 (2017). https://doi.org/10.1186/s12891-017-1805-9
Meng, Q., An, S., Damion, R.A., **, Z., Wilcox, R., Fisher, J., Jones, A.: The effect of collagen fibril orientation on the biphasic mechanics of articular cartilage. J. Mech. Behav. Biomed. Mater. 65, 439–453 (2017). https://doi.org/10.1016/J.JMBBM.2016.09.001
Ronkainen, A.P., Fick, J.M., Herzog, W., Korhonen, R.K.: Site-specific cell-tissue interactions in rabbit knee joint articular cartilage. J. Biomech. 49, 2882–2890 (2016). https://doi.org/10.1016/J.JBIOMECH.2016.06.033
Alhadlaq, H.A., **a, Y., Moody, J.B., Matyas, J.R.: Detecting structural changes in early experimental osteoarthritis of tibial cartilage by microscopic magnetic resonance imaging and polarised light microscopy. Ann. Rheum. Dis. 63, 709–717 (2004). https://doi.org/10.1136/ARD.2003.011783
Oloyede, A., Flachsmann, R., Broom, N.: The dramatic influence of loading velocity on the compressive response of articular cartilage. Connect. Tissue Res. 27, 211–224 (1992)
Hayes, W.C., Bodine, A.J.: Flow-independent viscoelastic properties of articular cartilage matrix. J. Biomech. 11, 407–419 (1978). https://doi.org/10.1016/0021-9290(78)90075-1
Ahsanizadeh, S., Li, L.P.: Strain-rate-dependent non-linear tensile properties of the superficial zone of articular cartilage. Connect. Tissue Res. 56, 469–476 (2015)
Acknowledgments
The present study was supported by the Natural Sciences and Engineering Research Council of Canada. The first indentation test of a porcine joint was performed at Biomomentum (Quebec, Canada) with a Mach-1 tester (the original photos for Figs. 3 and 5 were taken at Biomomentum and modified for using here with permission). All subsequent indentation tests and joint reconstructions were performed using Dr. Brent Edwards’ facility at the Human Performance Lab, where Andrew Sawatsky trained Daniel Tang for the use of Mach-1. The MRI images were obtained at the Centre for Mobility and Joint Health, Dr. Steven Boyd’s lab at the University of Calgary.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Zare, M., Tang, D., Li, L. (2020). Poromechanical Modeling of Porcine Knee Joint Using Indentation Map of Articular Cartilage. In: Ateshian, G., Myers, K., Tavares, J. (eds) Computer Methods, Imaging and Visualization in Biomechanics and Biomedical Engineering. CMBBE 2019. Lecture Notes in Computational Vision and Biomechanics, vol 36. Springer, Cham. https://doi.org/10.1007/978-3-030-43195-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-43195-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-43194-5
Online ISBN: 978-3-030-43195-2
eBook Packages: EngineeringEngineering (R0)