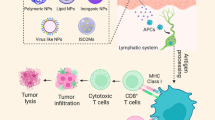
Abstract
Nanoparticles have the capacity to activate the immune system, based on both intrinsic particle characteristics and through the delivery of immune activating cargo. Co-delivery of antigens with adjuvants, such as cytokines, cytotoxic agents or pathogen-associated molecular patterns, presents opportunities for stimulating antigen-specific immune responses. This chapter highlights the immunogenic benefits of select chemotherapeutics and the use of nanoparticles to deliver immunogenic molecules and antigens. The influence of intrinsic nanoparticle properties and biological barriers on immune responses to nanoparticles is also discussed. In closing, a summary of nanoparticles approved for clinical use in the United States and examples of those approved in other countries are presented to highlight successes in nano-immunotherapy.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Strassburg MA. The global eradication of smallpox. Am J Infect Control. 1982;10(2):53–9.
World Health Organization. Global vaccine action plan: monitoring eaaarGW.
Hinman A. Eradication of vaccine-preventable diseases. Annu Rev Public Health. 1999;20:211–29.
Powell BS, Andrianov AK, Fusco PC. Polyionic vaccine adjuvants: another look at aluminum salts and polyelectrolytes. Clin Exp Vaccine Res. 2015;4(1):23–45.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801.
Serda RE. Particle platforms for cancer immunotherapy. Int J Nanomedicine. 2013;8:1683–96.
Powell BS, Andrianov AK, Fusco PC. Polyionic vaccine adjuvants: another look at aluminum salts and polyelectrolytes. Clinical and experimental vaccine research. 2015;4(1):23–45.
De Gregorio E, Rappuoli R. From empiricism to rational design: a personal perspective of the evolution of vaccine development. Nat Rev Immunol. 2014;14(7):505–14.
Di Pasquale A, Preiss S, Tavares Da Silva F, Garcon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines (Basel). 2015;3(2):320–43.
Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10(8):2147–62.
Fuenmayor J, Godia F, Cervera L. Production of virus-like particles for vaccines. New Biotechnol. 2017;39(Pt B):174–80.
Sahdev P, Ochyl LJ, Moon JJ. Biomaterials for nanoparticle vaccine delivery systems. Pharm Res. 2014;31(10):2563–82.
Meraz IM, Savage DJ, Segura-Ibarra V, et al. Adjuvant cationic liposomes presenting MPL and IL-12 induce cell death, suppress tumor growth, and alter the cellular phenotype of tumors in a murine model of breast cancer. Mol Pharm. 2014;11(10):3484–91.
Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811.
Joshi MD, Unger WJ, Storm G, van Kooyk Y, Mastrobattista E. Targeting tumor antigens to dendritic cells using particulate carriers. J Control Release. 2012;161(1):25–37.
Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316(5824):612–6.
Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425(6956):397–402.
Houde M, Bertholet S, Gagnon E, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425(6956):402–6.
Basha G, Lizee G, Reinicke AT, Seipp RP, Omilusik KD, Jefferies WA. MHC class I endosomal and lysosomal trafficking coincides with exogenous antigen loading in dendritic cells. PLoS One. 2008;3(9):e3247.
Croissant JG, Fatieiev Y, Omar H, et al. Periodic mesoporous organosilica nanoparticles with controlled morphologies and high drug/dye loadings for multicargo delivery in cancer cells. Chemistry. 2016;22(28):9607–15.
Noureddine A, Brinker CJ. Pendant/bridged/mesoporous silsesquioxane nanoparticles: versatile and biocompatible platforms for smart delivery of therapeutics. Chem Eng J. 2018;340:125–47.
Park J, Wrzesinski SH, Stern E, et al. Combination delivery of TGF-beta inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11(10):895–905.
Fan Y, Kuai R, Xu Y, Ochyl LJ, Irvine DJ, Moon JJ. Immunogenic cell death amplified by co-localized adjuvant delivery for cancer immunotherapy. Nano Lett. 2017;17(12):7387–93.
Dane EL, Irvine DJ. Big thinking for adjuvants. Nat Biotechnol. 2015;33(11):1146–8.
Meraz IM, Hearnden CH, Liu X, et al. Multivalent presentation of MPL by porous silicon microparticles favors T helper 1 polarization enhancing the anti-tumor efficacy of doxorubicin nanoliposomes. PLoS One. 2014;9(4):e94703.
Horisawa E, Kubota K, Tuboi I, et al. Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharm Res. 2002;19(2):132–9.
Oussoren C, Storm G. Liposomes to target the lymphatics by subcutaneous administration. Adv Drug Deliv Rev. 2001;50(1–2):143–56.
Mahony D, Cavallaro AS, Stahr F, Mahony TJ, Qiao SZ, Mitter N. Mesoporous silica nanoparticles act as a self-adjuvant for ovalbumin model antigen in mice. Small. 2013;9(18):3138–46.
Borges O, Cordeiro-da-Silva A, Romeijn SG, et al. Uptake studies in rat Peyer’s patches, cytotoxicity and release studies of alginate coated chitosan nanoparticles for mucosal vaccination. J Control Release. 2006;114(3):348–58.
Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem Rev. 2015;115(19):11109–46.
Wang J, Byrne JD, Napier ME, DeSimone JM. More effective nanomedicines through particle design. Small. 2011;7(14):1919–31.
Gutierro I, Hernandez RM, Igartua M, Gascon AR, Pedraz JL. Size dependent immune response after subcutaneous, oral and intranasal administration of BSA loaded nanospheres. Vaccine. 2002;21(1–2):67–77.
McClean S, Prosser E, Meehan E, et al. Binding and uptake of biodegradable poly-DL-lactide micro- and nanoparticles in intestinal epithelia. Eur J Pharm Sci. 1998;6(2):153–63.
Simecka JW. Mucosal immunity of the gastrointestinal tract and oral tolerance. Adv Drug Deliv Rev. 1998;34(2–3):235–59.
Jiang PL, Lin HJ, Wang HW, et al. Galactosylated liposome as a dendritic cell-targeted mucosal vaccine for inducing protective anti-tumor immunity. Acta Biomater. 2015;11:356–67.
Stano A, Nembrini C, Swartz MA, Hubbell JA, Simeoni E. Nanoparticle size influences the magnitude and quality of mucosal immune responses after intranasal immunization. Vaccine. 2012;30(52):7541–6.
Reddy ST, van der Vlies AJ, Simeoni E, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25(10):1159–64.
Sloat BR, Sandoval MA, Hau AM, He Y, Cui Z. Strong antibody responses induced by protein antigens conjugated onto the surface of lecithin-based nanoparticles. J Control Release. 2010;141(1):93–100.
Li X, Sloat BR, Yanasarn N, Cui Z. Relationship between the size of nanoparticles and their adjuvant activity: data from a study with an improved experimental design. Eur J Pharm Biopharm. 2011;78(1):107–16.
Kumar S, Anselmo AC, Banerjee A, Zakrewsky M, Mitragotri S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J Control Release. 2015;220(Pt A):141–8.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52.
Wang C, Ge Q, Ting D, et al. Molecularly engineered poly(ortho ester) microspheres for enhanced delivery of DNA vaccines. Nat Mater. 2004;3(3):190–6.
Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release. 2006;112(1):26–34.
Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38(5):1404–13.
Mueller SN, Tian S, DeSimone JM. Rapid and persistent delivery of antigen by lymph node targeting PRINT nanoparticle vaccine carrier to promote humoral immunity. Mol Pharm. 2015;12(5):1356–65.
Fifis T, Gamvrellis A, Crimeen-Irwin B, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173(5):3148–54.
Nishioka Y, Yoshino H. Lymphatic targeting with nanoparticulate system. Adv Drug Deliv Rev. 2001;47(1):55–64.
Mottram PL, Leong D, Crimeen-Irwin B, et al. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm. 2007;4(1):73–84.
Katare YK, Muthukumaran T, Panda AK. Influence of particle size, antigen load, dose and additional adjuvant on the immune response from antigen loaded PLA microparticles. Int J Pharm. 2005;301(1–2):149–60.
Jain AK, Goyal AK, Gupta PN, et al. Synthesis, characterization and evaluation of novel triblock copolymer based nanoparticles for vaccine delivery against hepatitis B. J Control Release. 2009;136(2):161–9.
Thomas C, Gupta V, Ahsan F. Influence of surface charge of PLGA particles of recombinant hepatitis B surface antigen in enhancing systemic and mucosal immune responses. Int J Pharm. 2009;379(1):41–50.
Thomas C, Gupta V, Ahsan F. Particle size influences the immune response produced by hepatitis B vaccine formulated in inhalable particles. Pharm Res. 2010;27(5):905–19.
Meraz IM, Melendez B, Gu J, et al. Activation of the Inflammasome and enhanced migration of microparticle-stimulated dendritic cells to the draining lymph node. Mol Pharm. 2012;9:2049–62.
Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–9.
Jung T, Kamm W, Breitenbach A, Kaiserling E, **ao JX, Kissel T. Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake? Eur J Pharm Biopharm. 2000;50(1):147–60.
Roux X, Dubuquoy C, Durand G, et al. Sub-nucleocapsid nanoparticles: a nasal vaccine against respiratory syncytial virus. PLoS One. 2008;3(3):e1766.
Yu JR, Kim S, Lee JB, Chang J. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. J Virol. 2008;82(5):2350–7.
Rodrigues TC, Oliveira MLS, Soares-Schanoski A, et al. Mucosal immunization with PspA (pneumococcal surface protein a)-adsorbed nanoparticles targeting the lungs for protection against pneumococcal infection. PLoS One. 2018;13(1):e0191692.
Kunda NK, Alfagih IM, Miyaji EN, et al. Pulmonary dry powder vaccine of pneumococcal antigen loaded nanoparticles. Int J Pharm. 2015;495(2):903–12.
Dhakal S, Renu S, Ghimire S, et al. Mucosal immunity and protective efficacy of intranasal inactivated influenza vaccine is improved by chitosan nanoparticle delivery in pigs. Front Immunol. 2018;9:934.
Stylianou E, Diogo GR, Pepponi I, et al. Mucosal delivery of antigen-coated nanoparticles to lungs confers protective immunity against tuberculosis infection in mice. Eur J Immunol. 2014;44(2):440–9.
Baleeiro RB, Schweinlin M, Rietscher R, et al. Nanoparticle-based mucosal vaccines targeting tumor-associated antigens to human dendritic cells. J Biomed Nanotechnol. 2016;12(7):1527–43.
Wang D, Molavi O, Lutsiak ME, Elamanchili P, Kwon GS, Samuel J. Poly(D,L-lactic-co-glycolic acid) microsphere delivery of adenovirus for vaccination. J Pharm Pharm Sci. 2007;10(2):217–30.
Maldonado-Contreras AL, McCormick BA. Intestinal epithelial cells and their role in innate mucosal immunity. Cell Tissue Res. 2011;343(1):5–12.
Moss DM, Curley P, Kinvig H, Hoskins C, Owen A. The biological challenges and pharmacological opportunities of orally administered nanomedicine delivery. Expert Rev Gastroenterol Hepatol. 2018;12(3):223–36.
Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31(1):51–72.
Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2006;13:54–54.
Panaretakis T, Kepp O, Brockmeier U, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28(5):578–90.
Martins I, Kepp O, Galluzzi L, et al. Surface-exposed calreticulin in the interaction between dying cells and phagocytes. Annals of the New York Academy of Sciences. 2010;1209(1):77–82.
Birge RB, Boeltz S, Kumar S, et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. 2016;23(6):962–78.
Wong DY, Ong WW, Ang WH. Induction of immunogenic cell death by chemotherapeutic platinum complexes. Angewandte Chemie (International ed. in English). 2015;54(22):6483–7.
Zhao X, Yang K, Zhao R, et al. Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials. 2016;102:187–97.
Lu J, Liu X, Liao YP, et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun. 2017;8(1):1811.
Zheng DW, Chen JL, Zhu JY, et al. Highly integrated Nano-platform for breaking the barrier between chemotherapy and immunotherapy. Nano Lett. 2016;16(7):4341–7.
Noureddine A, Lichon L, Maynadier M, et al. Controlled multiple functionalization of mesoporous silica nanoparticles: homogeneous implementation of pairs of functionalities communicating through energy or proton transfers. Nanoscale. 2015;7(26):11444–52.
Ambrogio MW, Thomas CR, Zhao YL, Zink JI, Stoddart JF. Mechanized silica nanoparticles: a new frontier in theranostic nanomedicine. Acc Chem Res. 2011;44(10):903–13.
Noureddine A, Gary-Bobo M, Lichon L, et al. Bis-clickable mesoporous silica nanoparticles: straightforward preparation of light-actuated Nanomachines for controlled drug delivery with active targeting. Chemistry. 2016;22(28):9624–30.
Croissant J, Zink JI. Nanovalve-controlled cargo release activated by plasmonic heating. J Am Chem Soc. 2012;134(18):7628–31.
Saint-Cricq P, Deshayes S, Zink JI, Kasko AM. Magnetic field activated drug delivery using thermodegradable azo-functionalised PEG-coated core-shell mesoporous silica nanoparticles. Nanoscale. 2015;7(31):13168–72.
Roy A, Singh MS, Upadhyay P, Bhaskar S. Combined chemo-immunotherapy as a prospective strategy to combat cancer: a nanoparticle based approach. Mol Pharm. 2010;7(5):1778–88.
Roy A, Chandra S, Mamilapally S, Upadhyay P, Bhaskar S. Anticancer and immunostimulatory activity by conjugate of paclitaxel and non-toxic derivative of LPS for combined chemo-immunotherapy. Pharm Res. 2012;29(8):2294–309.
Seth A, Heo MB, Lim YT. Poly (gamma-glutamic acid) based combination of water-insoluble paclitaxel and TLR7 agonist for chemo-immunotherapy. Biomaterials. 2014;35(27):7992–8001.
Peabody DS, Manifold-Wheeler B, Medford A, Jordan SK, do Carmo Caldeira J, Chackerian B. Immunogenic display of diverse peptides on virus-like particles of RNA phage MS2. J Mol Biol. 2008;380(1):252–63.
Tumban E, Peabody J, Peabody DS, Chackerian B. A universal virus-like particle-based vaccine for human papillomavirus: longevity of protection and role of endogenous and exogenous adjuvants. Vaccine. 2013;31(41):4647–54.
Tyler M, Tumban E, Peabody DS, Chackerian B. The use of hybrid virus-like particles to enhance the immunogenicity of a broadly protective HPV vaccine. Biotechnol Bioeng. 2014;111(12):2398–406.
Saboo S, Tumban E, Peabody J, et al. Optimized formulation of a thermostable spray-dried virus-like particle vaccine against human papillomavirus. Mol Pharm. 2016;13(5):1646–55.
HAHN TJ, WEBB B, KUTNEY J, et al. Rapid manufacture and release of a GMP batch of Zaire ebolavirus glycoprotein vaccine made using recombinant Baculovirus-Sf9 insect cell culture technology. Bioprocess J. 2015;14(1):6–14.
Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioengineering & translational medicine. 2016;1(1):10–29.
Fries L, Shinde V, Stoddard JJ, et al. Immunogenicity and safety of a respiratory syncytial virus fusion protein (RSV F) nanoparticle vaccine in older adults. Immun Ageing. 2017;14(1):8.
Colosia AD, Yang J, Hillson E, et al. The epidemiology of medically attended respiratory syncytial virus in older adults in the United States: a systematic review. PLoS One. 2017;12(8):e0182321.
August A, Glenn GM, Kpamegan E, et al. A phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine. 2017;35(30):3749–59.
Glenn GM, Fries LF, Smith G, et al. Modeling maternal fetal RSV F vaccine induced antibody transfer in Guinea pigs. Vaccine. 2015;33(47):6488–92.
Smith G, Liu Y, Flyer D, et al. Novel hemagglutinin nanoparticle influenza vaccine with matrix-M™ adjuvant induces hemagglutination inhibition, neutralizing, and protective responses in ferrets against homologous and drifted a (H3N2) subtypes. Vaccine. 2017;35(40):5366–72.
Liu YV, Massare MJ, Pearce MB, et al. Recombinant virus-like particles elicit protective immunity against avian influenza a (H7N9) virus infection in ferrets. Vaccine. 2015;33(18):2152–8.
Fries LF, Smith GE, Glenn GM. A recombinant viruslike particle influenza A (H7N9) vaccine. N Engl J Med. 2013;369(26):2564–6.
TJ HAHN, COURBRON D, HAMER M, MASOUD M, WONG J, TAYLOR K. Rapid manufacture and release of a GMP batch of avian influenza a (H7N9) virus-like particle vaccine made using recombinant baculovirus-Sf9 insect cell culture technology. BioProcessing. 2013;12(2):1538–8786.
Smith GE, Flyer DC, Raghunandan R, et al. Development of influenza H7N9 virus like particle (VLP) vaccine: homologous a/Anhui/1/2013 (H7N9) protection and heterologous a/chicken/Jalisco/CPA1/2012 (H7N3) cross-protection in vaccinated mice challenged with H7N9 virus. Vaccine. 2013;31(40):4305–13.
Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med. 2018;378:2346–8.
Bengtsson KL, Song H, Stertman L, et al. Matrix-M adjuvant enhances antibody, cellular and protective immune responses of a Zaire Ebola/Makona virus glycoprotein (GP) nanoparticle vaccine in mice. Vaccine. 2016;34(16):1927–35.
Raghunandan R, Lu H, Zhou B, et al. An insect cell derived respiratory syncytial virus (RSV) F nanoparticle vaccine induces antigenic site II antibodies and protects against RSV challenge in cotton rats by active and passive immunization. Vaccine. 2014;32(48):6485–92.
Coleman CM, Liu YV, Mu H, et al. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32(26):3169–74.
Reimer JM, Karlsson KH, Lövgren-Bengtsson K, Magnusson SE, Fuentes A, Stertman L. Matrix-M™ adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen. PLoS One. 2012;7(7):e41451.
Thomas DN. RSV-F vaccine and influenza vaccine co-administration study in the elderly. 2014.
Sridhar S. Clinical development of Ebola vaccines. Therapeutic advances in vaccines. 2015;3(5–6):125–38.
Grippin AJ, Sayour EJ, Mitchell DA. Translational nanoparticle engineering for cancer vaccines. Oncoimmunology. 2017;6(10):e1290036.
Alamoudi K, Martins P, Croissant JG, Patil S, Omar H, Khashab NM. Thermoresponsive pegylated bubble liposome nanovectors for efficient siRNA delivery via endosomal escape. Nanomedicine. 2017;12(12):1421–33.
Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A. Application of liposomes in medicine and drug delivery. Artificial cells, nanomedicine, and biotechnology. 2016;44(1):381–91.
Yaroslavov AA, Efimova AA, Sybachin AV, Chvalun SN, Kulebyakina AI, Kozlova EV. Biodegradable multi-liposomal containers. RSC Adv. 2015;5(40):31460–4.
Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10(1):975–99.
Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non–small-cell lung cancer. J Clin Oncol. 2005;23(27):6674–81.
Kruit WH, Suciu S, Dreno B, et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer melanoma Group in Metastatic Melanoma. J Clin Oncol. 2013;31(19):2413–20.
Berinstein NL, Karkada M, Morse MA, et al. First-in-man application of a novel therapeutic cancer vaccine formulation with the capacity to induce multi-functional T cell responses in ovarian, breast and prostate cancer patients. J Transl Med. 2012;10(1):156.
Berinstein NL, Karkada M, Oza AM, et al. Survivin-targeted immunotherapy drives robust polyfunctional T cell generation and differentiation in advanced ovarian cancer patients. Oncoimmunology. 2015;4(8):e1026529.
Kitano S, Kageyama S, Nagata Y, et al. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin Can Res. 2006;12(24):7397–405.
Wada H, Sato E, Uenaka A, et al. Analysis of peripheral and local anti-tumor immune response in esophageal cancer patients after NY-ESO-1 protein vaccination. Int J Cancer. 2008;123(10):2362–9.
Maraskovsky E, Schnurr M, Wilson NS, Robson NC, Boyle J, Drane D. Development of prophylactic and therapeutic vaccines using the ISCOMATRIX adjuvant. Immunol Cell Biol. 2009;87(5):371–6.
Speiser DE, Schwarz K, Baumgaertner P, et al. Memory and effector CD8 T-cell responses after nanoparticle vaccination of melanoma patients. J Immunother. 2010;33(8):848–58.
Bendandi M, Gocke CD, Kobrin CB, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte–monocyte colony-stimulating factor against lymphoma. Nat Med (NY, NY, US). 1999;5(10):1171.
Altin J, Atmosukarto I, De Wildt RM, Parish C, Price J. Composition for targeting dendritic cells. Google Patents; 2014.
Kranz LM, Diken M, Haas H, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401.
Thomas DN. Safety and immunogenicity of the RSV-F vaccine in older adults previously treated with the same vaccine or placebo in the prior year. 2017.
Thomas DN. Study to evaluate the immunogenicity and safety of an Ebola virus (EBOV) glycoprotein (GP) vaccine in healthy subjects. 2016.
Thomas DN. Evaluation of the safety and immunogenicity of a recombinant trivalent nanoparticle influenza vaccine with matrix M-1 adjuvant (NanoFlu). 2018.
Thomas DN. A Phase I Randomized, Observer-Blinded, Dose-Ranging Study in Healthy Subjects 24 to <72 Months of Age. 2016.
Thomas DN. A study to determine the safety and efficacy of the RSV F vaccine to protect infants via maternal immunization. 2018.
Thomas DN. RSV F Vaccine maternal immunization study in healthy third-trimester pregnant women. 2017.
Thomas DN. RSV-F vaccine dose ranging study in young women. 2014.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 American Association of Pharmaceutical Scientists
About this chapter
Cite this chapter
Noureddine, A., Croissant, J.G., Davis, H.O., Friedrich, L.I., Serda, R.E. (2020). Nanoparticle Vaccines for Immunotherapy: From Design to Clinical Trials. In: Muttil, P., Kunda, N. (eds) Mucosal Delivery of Drugs and Biologics in Nanoparticles. AAPS Advances in the Pharmaceutical Sciences Series, vol 41. Springer, Cham. https://doi.org/10.1007/978-3-030-35910-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-35910-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35909-6
Online ISBN: 978-3-030-35910-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)