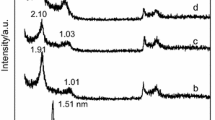
Abstract
Vermiculite is an Alumino-Silicate clays which is one of the layered materials that can form intercalation compounds [1–3]. The host layer of Vermiculite is classified as a 2:1 layered silicate due to its structure [2]. This 2:1 layered silicate is responsible for several of the distinctive properties of Vermiculite. First, unlike graphite layers which are charge neutral, 2:1 layered silicate has a negative layer charge. To compensate this the galleries of Vermiculite are occupied by cations and in turn this makes the intercalation process in CIC’s (Clay Intercalation Compounds) an ion exchange process. Second, because the 2:1 silicate layers are composed of multiple, cross-linked planes of atoms, one can expect the clay layers to be relatively rigid to transverse distortions. The rigidity of the silicate layer is important not only for the study of fundamental physical properties of quasi-two dimensional systems but also for practical applications such as catalysis [4, 5]. In any case, it is very important to know how rigid the layer is and what factors affect layer rigidity.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others

References
G.W. Brindly and G. Brown (eds.): Crystal Structure of Clay Minerals and Their X-Ray Identification (Minerological Society, London, 1980).
R.E. Grim: Clay Minerology (McGraw-Hill, New York, 1968).
S.A. Solin: J. Molec. Catalysis 27, 293 (1984).
T.J. Pinnavaia: Science 220, 365 (1983).
M.S. Wittingham and A.J. Jacobson (eds.): Intercalation Chemistry Article by J.M. Thomas (Academic Press, New York, 1982).
H. Kim and T.J. Pinnavaia: unpublished.
B.R. York, S.A. Solin, N. Wada, R.H. Raythatha, I.D. Johnson, and T.J. Pinnavaia: Solid State Comm. 54 475 (1985).
M.S. Dresselhaus and G. Dresselhaus: Adv. Phys. 30, 139 (1981).
D.H. Fink, F.S. Nakayama, and B.L. McNeal: Soil Sci. Soc. Amer. Proc. 35, 552 (1971)
M.B. McBride and M.M. Mortland: Clays and Clay Minerals 21, 323 (1973).
S.B. Hendricks and E. Teller: J. Chem. Phys. 10, 147 (1942).
M.S. Dresselhaus (ed.): Intercalation in Layered Materials, NATO ASI Series 148, Article by S.A. Solin (Plenum Press, New York, 1986).
M. Ishii, T. Shimanouchi, and M. Nakahirs: Inorganica Chimica Acta /1:3/, 387 (1967).
D.R. Hines, N. Wada, and M. Suzuki: Bull. Am. Phys. Soc. 32, 559 (1987)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1987 Springer Science+Business Media New York
About this chapter
Cite this chapter
Lee, S., Kim, H., Solin, S.A., Pinnavaia, T.J. (1987). Layer rigidity of clay intercalation compound: [Me4N+]1−x [Me3NH+]x −V. In: Legrand, A.P., Flandrois, S. (eds) Chemical Physics of Intercalation. NATO ASI Series, vol 172. Springer, Boston, MA. https://doi.org/10.1007/978-1-4757-9649-0_44
Download citation
DOI: https://doi.org/10.1007/978-1-4757-9649-0_44
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4757-9651-3
Online ISBN: 978-1-4757-9649-0
eBook Packages: Springer Book Archive