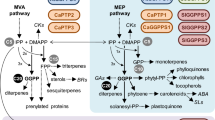
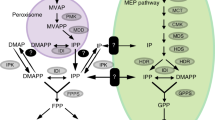
Abstract
There is hardly a single process in the living plant cell that does not require the biochemical and physiological involvement of isoprenoids. It is thus of great theoretical as well as practical interest to have a profound knowledge of how those various classes of compounds are synthesized, what the properties of the enzymes participating are and how this synthesis is regulated by various factors depending on the demand of the develo** and mature plant for various classes of isoprenoids and prenyllipids. Although a large body of information is available on the structures of the myriad of isoprenoids occurring in plants isolated so far, many of the details to be discussed in this contribution have frequently been learned through experiments first performed with animal cells or yeast, systems that, at least as far as the variety of isoprenoid end products is concerned, are not as complex as plants. Therefore, an important purpose of this article is to consider the feasibility of regulatory and of other models developed for non-plant systems that might be likely to explain some features of the enzymology of isoprenoid biosynthesis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
KLOSTERMAN, H.J., SMITH, F. 1954. The isolation of β-hydroxy-β-methylglutaric acid from the seed of flax. J. Am. Chem. Soc. 76: 1229–1230.
JOHNSTON, J.A., RACUSEN, D.W., BONNER, J. 1954. The metabolism of isoprenoid precursors in a plant system. Proc. Natl. Acad. Sci. U.S.A. 40: 1031–1037
RUDNEY, H. 1955. The synthesis of β,β-dimethylacrylic acid in rat liver homogenates. J. Am. Chem. Soc 77: 1698–1699.
RUDNEY, H. 1957. The biosynthesis of β-hydroxy-β- methylglutaric acid. J. Biol. Chem. 227: 363–377
RUDNEY, H. 1957. The biosynthesis of β-hydroxy-β-methylglutaryl coenzyme A. J. Am. Chem. Soc 79:5580–5581.
RUDNEY, H., FERGUSON, J.J. 1959. The biosynthesis of β-hydroxy-β-methylglutaryl coenzyme A in yeast. II. The formation of hydroxymethylglutaryl coenzyme A via the condensation of acetyl-coenzyme A and acetoacetyl coenzyme A in yeast. J. Biol. Chem. 234: 1076–1080
LYNEN, F., HENNING, U., BUBLITZ, C., SORBO, B., KRÖPLIN-RUEFF, L. Der chemische Mechanismus der Acetessig-säurebildung in der Leber. Biochem. Z. 330: 269–295
WOLF, D.E., HOFFMAN, C.H., ALDRICH, P.E., SKEGGS, H. R., WRIGHT, L.D., FOLKERS, K. 1956. β-Hydroxy-β-methyl δ-valerolactone (divalonic acid), a new biological factor. J. Amer. Chem. Soc. 78: 4499
TAVORMINA, P.A., GIBBS, M.H. 1956. The metabolism of β, δ-dihydroxy-β-methylvaleric acid by liver homogenates. J. Am. Chem. Soc. 78–6210
TAVORMINA, P.A., GIBBS, M.H., HUFF, J.W. 1956. The utilization of β-hydroxy-β-methyl-δ-valerolactone in cholesterol biosynthesis. J. Am. Chem. Soc. 78: 4498
LYNEN, F., GRASSL, M. 1958. Darstellung von (-)-Mevalonsäure durch bakterielle Racematspaltung. Blochem. Z. 335: 123–127.
GOODWIN, T. W. (ed.) 1970. Natural substances formed biologically from mevalonic acid. Biochem. Soc. Symp. No. 29, Liverpool 1969, Academic Press, New York.
NES, W.R., MCKEAN, M.L. 1977. Biochemistry of steroids and other isopentenoids. University Park Press, Baltimore — London — Tokyo.
PORTER, J., SPURGEON, S.L. (eds.) 1981. Biosynthesis of Isoprenoid Compounds, Vol. 1, John Wiley and Sons, New York — Chichester — Brisbane — Toronto.
FERGUSON, J.J., DURR, I.F., RUDNEY, H. 1959. The biosynthesis of mevalonic acid. Proc. Natl. Acad. Sci. U.S.A. 45: 499–504.
RUDNEY, H. The biosynthesis of β-hydroxy-β-methyl-glutaryl coenzyme A and its conversion to mevalonic acid. In: Ciba Foundation Symposium on the Biosynthesis of Terpenes and Sterols. (G E.W. Wolstenholme, M. O’Connor, eds.), J.A. Churchill, London, pp. 75–94.
KIRTLEY, M.E., RUDNEY, H. 1967. Some properties and mechanism of action of the β-hydroxy-β-methylglutaryl coenzyme A reductase of yeast. Biochemistry U.S.A. 6: 230–238.
LYNEN, F. New aspects of acetate incorporation into isoprenoid precursors. In: Ciba Foundation Symposium on the Biosynthesis of Terpenes and Sterols. (G.E.W. Wolstenholme, M. O’Connor, eds.), J.A. Churchill, London, pp. 95–118.
KNAPPE, J., RINGELMANN, E., LYNEN, F. 1959. Über die β-Hydroxy-β-methylglutaryl Reduktase der Hefe. Zur Biosynthese der Terpene IX. Biochem. Z. 332: 195–213.
BRODIE J., PORTER, J.W. 1960. The synthesis of mevalonic acid by non-particulate avian and mammalian enzyme systems. Biochim. Biophys. Res. Commun. 3: 173–177.
SPURGEON, S.I., PORTER, J.W. 1981. Introduction. In: Biosynthesis of Isoprenoid Compounds. (J.W. Porter, S.L. Spurgeon, eds.), Vol. 1, John Wiley and Sons, New York — Chichester — Brisbane — Toronto, pp. 1–46.
QURESHI, N., PORTER, J.W. 1981. Conversion of acetyl-coenzyme A to isopentenyl pyrophosphate. In: Biosynthesis of Isoprenoid Compounds. (J.W. Porter, S.L. Spurgeon, eds.), Vol. 1, John Wiley and Sons, New York — Chichester — Brisbane — Toronto, pp. 47–94.
DUGAN, R.E. 1981. Regulation of HMG-CoA reductase. In: Biosynthesis of Isoprenoid Compounds. (J.W. Porter, S.L. Spurgeon, eds.), Vol. 1, John Wiley and Sons, New York — Chichester — Brisbane — Toronto, pp. 95–159.
SABINE, J.R. 1983. Monographs on enzyme biology: HMG-CoA reductase. (J.R. Sabine, ed.), CRC Press, Boca Raton.
PREISS, B. 1985. Regulation of HMG-CoA reductase. (B. Preiss, ed.), Academic Press, New York.
BACH, T.J. 1987. Synthesis and metabolism of mevalonic acid in plants. Plant Physiol. Biochem. 25: 163–178.
GRAY, J.C. 1987. Control of isoprenoid biosynthesis in higher plants. Adv. Bot. Res. 14: 25–91.
BROOKER, J.D., RUSSELL, D.W. 1975. Properties of microsomal 3-hydroxy-3-methylglutaryl-coenzyme A reductase from Pisum sativum seedlings. Arch. Biochem. Biopys. 167: 723–729.
BROOKER, J.D., RUSSELL, D.W. 1979. Regulation of microsomal 3-hydroxy-3-methylglutaryl-coenzyme A reductase from pea seedlings. Rapid post-translational phytochrome-mediated decrease in activity and in vivo regulation by isoprenoid products. Arch. Biophys. Biochem. 198: 323–334.
BACH, T.J., LICHTENTHALER, H.K., RÉTEY, J. 1980. Properties of membrane-bound 3-hydroxy-3-methylglutaryl-coenzyme A reductase from radish seedlings and some aspects of its regulation. In: Biogenesis and Function of Plant Lipids. (P. Mazliak et al., eds.), Elsevier, Amsterdam, pp. 355–362.
WONG, R.J., MCCORMACK, D.K., RUSSELL, D.W. 1982. Plastid 3-hydroxy-3-methylglutaryl-coenzyme A reductase has distinctive kinetic and regulatory features: properties of the enzyme and positive phytochrome control of activity in pea seedlings. Arch. Biophys. Biochem. 216: 613–638.
RUSSELL, D.W., KNIGHT, J.S., WILSON, T.M. 1985. Pea seedling HMG-CoA reductase: regulation of activity in vitro by phosphorylation and Ca2+, and posttrans-lational control in vivo by phytochrome and isoprenoid hormones. Curr. Top. Plant Biochem. Physiol. 4: 191–206.
GRUMBACH, K.H., BACH, T.J. 1979. The effect of PSII herbicides, amitrol and SAN 67 06 on the activity of 3-hydroxy-3-methylglutaryl-coenzyme A reductase and the incorporation of (2–14C) acetate and (2-3H) mevalonate into chloroplast pigments of radish seedlings. Z. Naturforsch. 34c: 941–943.
RUSSELL, D.W., DAVIDSON, H. 1982. Regulation of cytosolic HMG-CoA reductase activity in pea seedlings: contrasting responses to different hormones, and hormone-product interaction, suggest hormonal modulation of activity. Biochem. Biophys. Res. Commun. 104: 1537–1543.
BACH, T.J. 1981. Untersuchungen zur Charakterisierung und Regulation der 3-Hydroxy-3-methylglutaryl-Coenzym A Reduktase (Mevalonat: NADP+ Oxidoreduktase, CoA acylierend, E.C. 1.1.1.34) in Keimlingen von Raphanus sativus.- Karlsr. Beitr. Pflanzenphysiol. (Karlsr. Contrib. Plant Physiol. ISSN 0173–3133) 10: 1–219.
BACH, T.J., LICHTENTHALER, H.K. 1984. Application of modified Lineweaver-Burk plots to studies of kinetics and regulation of radish 3-hydroxy-3-methylglutaryl-CoA reductase from radish seedlings. Biochim. Biophys. Acta 794: 152–161.
ISA, R.B.M., SIPAT, A.B. 1982. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase of Hevea latex: The occurrence of a heat-stable activator in the C-serum. Biochem. Biophys. Res. Commun. 108: 206–212.
SUZUKI, H., URITANI, I., OBA, K. 1975. The occurrence of and properties of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in sweet potato roots infected by Ceratocystis fimbriata. Physiol. Plant Pathol. 7: 265–276.
SUZUKI, K., OBA, K., URITANI, I. 1976. Subcellular localization of 3-hydroxy-3-methylglutaryl-coenzyme A reductase and other membrane-bound enzymes in sweet potato roots. Plant Cell Physiol. 17: 691–700.
ITO, R., OBA, K., URITANI, I. 1979. Mechanisms for the induction of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in HgCl2-treated sweet potato root tissue. PlantCell Physiol. 20: 867–874.
OBA, K., KONDO, K., DOKE, N., URITANI, I. 1985. Induction of 3-hydroxy-3-methylglutaryl CoA reductase in potato after slicing, fungal infection or chemical treatment, and some properties of the enzyme. Plant Cell Physiol. 26: 873–880.
STERMER, B.A., BOSTOCK, R.M. 1987. Involvement of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase in the regulation of sesquiterpenoid phytoalexin synthesis in potato. Plant Physiol. 84: 404–408.
CHAPPELL, J., NABLE, R. 1987. Induction of sesquiterpenoid biosynthesis in tobacco cell suspension cultures by fungal elicitor. Plant Physiol. 85: 469–473.
LEUBE, J., GRISEBACH, H. 1983. Further studies on induction of enzymes of phytoalexin synthesis in soybean and cultured soybean cells. Z. Naturforsch. 38c: 730–735.
VöGELI, U., CHAPPELL, J. 1988. Induction of sesquiterpene cyclase and suppression of squalene synthetase activities in plant cell cultures treated with fungal elicitor. Plant Physiol. 88: 1291–1296.
NARITA, J.O., GRUISSEM, W. 1989 Tomato hydroxymethyl-glutaryl-CoA reductase is required early in fruit development but not during fruit ripening. Plant Cell 1: 181–190.
LYNEN, F. 1967. Biosynthesis pathways from acetate to natural products. Activity of the enzymes in rubber synthesis. Pure Appl. Chem. 14: 137–167.
HEPPER, C.M., AUDLEY, B.G. 1969. The biosynthesis of rubber from β-hydroxy-β-methylglutaryl-coenzyme A in Hevea brasiliensis latex. Biochem. J. 114: 379–386.
WITITSUWANNAKUL, R. 1986. Diurnal variation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in latex of Hevea brasiliensis and its relation to rubber content. Experientia 42: 44–46.
SIPAT, A.B. 1982. Hydroxymethylglutaryl CoA reductase (NADPH) in the latex of Hevea brasiliensis. Phytochemistry 21: 2613–2618.
SIPAT, A. 1985. 3-Hydroxy-3-methylglutaryl-CoA reductase in the latex of Hevea brasiliensis. Meth. Enzymol. 110: 40–51.
BROOKER, J.D., RUSSELL, D.W. 1975. Subcellular localization of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in Pisum sativum seedlings. Arch. Biochem. Biophys. 67: 730–737.
SUZUKI, H., URITANI, I. 1977. Effects of bovine serum albumin and phospholipids on activity of microsomal 3-hydroxy-3-methylglutaryl-coenzyme A reductase in sweet potato roots. Plant Cell Physiol. 18: 485–495.
YU-ITO, R., OBA, K., URITANI, I. 1982. Some problems in the assay method of HMG-CoA reductase activity in sweet potato in the presence of other HMG-CoA utilizing enzymes. Agric. Biol. Chem. 46: 2087–2091.
DOUGLAS, T.J., PALEG, L.G. 1978. Amo 1618 effects on the incorporation of 14C-MVA and 14C-acetate into sterol in Nicotiana and Digitalis seedlings and cell-free preparations from Nicotiana. Phytochemistry 17: 713–718.
BACH, T.J. 1986. Hydroxymethylglutaryl-CoA reductase, a key enzyme in phytosterol synthesis? Lipids 21: 82–88.
BACH, T.J., ROGERS, D.H., RUDNEY, H. 1986. Detergent-solubilization, purification, and characterization of 3-hydroxy-3-methylglutaryl-CoA reductase from radish seedlings. Eur. J. Biochem. 154: 103–111.
CAMARA, B., BARDAT, F., DOGBO, O., BRANGEON, J., MONéGER, R. 1983. Terpenoid metabolism in plastids. Isolation and biochemical characteristics of Capsicum annuum chromoplasts. Plant Physiol. 73: 94–99.
NISHI, A., TSURITANI, I. 1983. Effect of auxin on the metabolism of mevalonic acid in suspension-cultured carrot cells. Phytochemistry 22: 399–401.
BONHOFF, A., LOYAL, R., FELLER, K., EBEL, J., GRISEBACH, H. 1986. Further investigations of race: cultivar-specific induction of enzymes related to phytoalexin biosynthesis in soybean roots following infection with Phytophthora megasperma f.sp. glycinea. Biol. Chem. Hoppe-Seyler 367: 797–802.
AREBALO, R.E., MITCHELL JR., E.D. 1984. Cellular distribution of 3-hydroxy-3-methylglutaryl coenzyme A reductase and mevalonate kinase in leaves of Nepeta cataria. Phytochemistry 23: 13–18.
KREUZ, K., KLEINIG, H. 1984. Synthesis of prenyl lipids in cells of spinach leaf. Compartmentation of enzymes for formation of isopentenyl diphosphate. Eur. J. Biochem. 141: 531–535.
CECCARELLI, N., LORENZI, R. 1984. Growth inhibition by competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase in Helianthus tuberosus tissue expiants. Plant Sci. Lett. 34: 269–276.
KONDO, K., OBA, K. 1986. Purification and characterization of 3-hydroxy-3-methylglutaryl CoA reductase from potato tubers. J. Biochem. Tokyo 100: 967–974.
SKRUKRUD, C.L., TAYLOR, S.E., HAWKINS, D.R., CALVIN, M. 1987. Triterpenoid biosynthesis in Euphorbia lathyris. In: The Metabolism, Structure, and Function of Plant Lipids. (P. K. Stumpf, J. B. Mudd, W. D. Nes, eds.), Plenum Press, New York — London, pp. 115–118.
SKRUKRUD, C.L., TAYLOR, S.E., HAWKINS, D.R., NEMETHY, E.K., CALVIN, M. 1988. Subcellular fractionation of triterpenoid biosynthesis in Euphorbia lathyris latex. Physiol. Plant. 74: 306–316.
LüTKE-BRINKHAUS, F., KLEINIG, H. 1987. Formation of isopentenyl diphosphate via mevalonate does not occur within etioplasts and etiochloroplasts of mustard (Sinapis alba L.) seedlings. Planta 171: 406–411.
RAMACHANDRA REDDY, A., DAS, V. S. R. 1986. Partial purification and characterization of 3-hydroxy-3-methylglutaryl coenzyme A reductase from the leaves of guayule (Parthenium argentatum). Phytochemistry 25: 2471–2474.
RAMACHANDRA REDDY, A., DAS, V.S.R. 1987. Chloroplast autonomy for the biosynthesis of isopentenyl diphosphate in guayule (Parthenium argentatum Gray). New. Phytol. 106: 457–464.
BOLL, M., KARDINAL, A., BERNDT, J. 1987. Properties and regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase from spruce (Picea abies). Biol. Chem. Hoppe-Seyler 368: 1024.
BOLL, M., MEßNER, B., KARDINAL, A. 1988. Regulation of phenylalanine ammonia-lyase (PAL) and 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) in spruce (Picea abies). Biol. Chem. Hoppe-Seyler 369: 799.
MAUREY, K.M., GOLBECK, J.H. 1985. Modulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR) activity in Ochromonas malhamensis. Plant Physiol. 11 (supplement): 45.
MAUREY, K., WOLF, F., GOLBECK, J. 1986. 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity in Ochromonas malhamensis. A system to study the relationship between enzyme activity and rate of steroid biosynthesis. Plant Physiol. 82: 523–527.
GOLBECK, J.H., MAUREY, K. M., NEWBERRY, G.A. 1985. Detection of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR) activity in Dunaliella salina. Plant Physiol. 11 (supplement): 48.
ROGERS, D.H., PANINI, S.R., RUDNEY, H. 1980. Rapid, high-yield purification of rat liver 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase. Anal. Biochem 101: 107–111.
BACH, T.J., RUDNEY, H. 1983 Solubilization and partial purification of membrane-bound HMG-CoA reductase from a plant source. J. Lipid Res. 24: 1404–1405.
BACH, T.J., ROGERS, D.H., RUDNEY, H. 1984. Purification and properties of membrane-bound 3-hydroxy-3-methylglutaryl-CoA reductase from radish seedlings. In: Structure, Function and Metabolism of Plant Lipids. (P.A. Siegenthaler and W. Eichenberger, eds.), Elsevier Science Publishers BV, Amsterdam, pp. 221–224.
QURESHI, N., DUGAN, R.E., CLELAND, W.W., PORTER, J.W. 1976. Kinetic analysis of the individual reductive steps catalyzed by β-hydroxy-β-methylglutaryl coenzyme A reductase obtained from yeast. Biochemistry U.S.A. 15: 4191–4197.
BASSON, M.E., THORSNESS, M., FINER-MOORE, J., STROUD, R.M. RINE, J. 1988. Structural and functional conservation between yeast and human 3-hydroxy-3-methylglutaryl coenzyme A reductases, the rate-limiting enzyme of sterol biosynthesis. Mol. Cell. Biol. 8: 3797–3808.
CHIN, D.J., GIL, G., RUSSELL, D.W., LISCUM, L, LUSKEY, K.L., BASU, S.K., OKAYAMA, H., BERG, P., GOLDSTEIN, J.L., BROWN, M.S. 1984. Nucleotide sequence of HMG-CoA reductase, a glycoprotein of the endoplasmic reticulum. Nature (Lond.) 308: 613–617.
REYNOLDS, G.A., BASU, S.K., OSBORNE, T.F., CHIN, D.J., GIL, G., BROWN, M.S., GOLDSTEIN, J.L., LUSKEY, K.L. 1984. HMG-CoA reductase: A negatively regulated gene with unusual promoter and 5′ untranslated regions. Cell 38: 275–285.
BROWN, D.A., SIMONI, R.D. 1984. Biogenesis of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, an integral glycoprotein of the endoplasmic reticulum. Broc. Natl. Acad. Sci. U.S.A. 81: 1674–1678.
LISCUM, L., FINER-MOORE, J., STROUD, R.M., LUSKEY, K.L., BROWN, M.S., GOLDSTEIN, J.L. 1985. Domain structure of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a glycoprotein of the endoplasmic reticulum. J. Biol. Chem. 260: 522–530.
LUSKEY, K.L., STEVENS, B. 1985. Human 3-hydroxy-3-methylglutaryl coenzyme A reductase. Conserved domains responsible for catalytic activity and sterol-regulated degradation. J. Biol Chem. 260: 10271–12277.
WOODWARD, H.D., ALLEN, J.M.C., LENNARZ, W.L. 1988. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase of the sea urchin embryo. Deduced structure and regulatory properties. J. Biol. Chem. 263: 18411–18418.
GERTLER, F.B., CHIU, C.Y., RICHTER-MANN, L., CHIN, D. J. 1988. Developmental and metabolic regulation of the Drosophila melanogaster 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mol. Cell. Biol. 8: 2713–2721.
ROGERS, D.H., PANINI, S.R., RUDNEY, H. 1983. Properties of HMG-CoA reductase and its mechanism of action. In: 3-Hydroxy-3-methylglutaryl Coenzyme A Reductase. (J. R. Sabine, ed.), CRC Press, Inc., Boca Raton, Florida, pp. 58–75.
EDWARDS, P.A., KEMPNER, E.S., LAN, S.F., ERICKSON, S.K. 1985. Functional size of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase as determined by radiation inactivation. J. Biol. Chem. 260: 10278–10282.
NESS, G.C., PENDLETON, L.C., MCCREERY, M.J. 1988. In situ determination of the functional size of hepatic 3-hydroxy-3-methylglutaryl-CoA reductase by radiation inactivation analysis. Biochim. Biophys. Acta 953: 361–364.
EDWARDS, P.A., LAN, S.F., FOGELMAN, A.M. 1983. Alterations in the rates of synthesis and degradation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase produced by cholestyramine and mevinolin. J. Biol. Chem. 258: 10219–10222.
NESS, G.C., MCCREERY, M.J., SAMPLE, C.E., SMITH, M. PENDLETON, L.C. 1985. Sulhydryl/disulfide forms of rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase. J. Biol. Chem. 260: 16395–16399
KENNELLY, P.J., BRANDT, K.G., RODWELL, V.W. 1983. 3-Hydroxy-3-methylglutaryl-CoA reductase: Solubilization in the presence of proteolytic inhibitors, partial purification, and reversible phosphorylation-dephosphorylation. Biochemistry USA 22: 2784–2788
DOTAN, I., SHECHTER, I. 1985. Reduced glutathione in chinese hamster ovary cells protects against inactivation of 3-hydroxy-3-methylglutaryl coenzyme A reductase by 2-mercaptoethanol disulfide. J. Cell Physiol. 122: 14–20.
ROITELMAN, J., SHECHTER, I. 1984. Regulation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase Evidence for thiol-dependent modulation of enzyme activity. J. Biol. Chem. 259: 870–877.
NESS, G.C., EALES, S.J., PENDLETON, L.C., SMITH, M. 1985. Activation of rat liver microsomal 3-hydroxy-3-methylglutaryl coenzyme A reductase by NADPH. Effects of dietary treatments. J. Biol. Chem. 260: 12391–12393.
CAPPEL, R.E., GILBERT, H.F. 1988. Thiol/disulfide exchange between 3-hydroxy-3-methylglutaryl-CoA reductase and glutathione. A thermodynamically facile dithiol oxidation. J. Biol Chem. 263: 12204–12212.
CLELAND, W.W. 1963. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim. Biophys. Acta 67: 104–137.
BACH, T.J., LICHTENTHALER, H.K. 1983. Mechanisms of inhibition by mevinolin (MK 803) of microsome-bound radish and of partially purified yeast HMG-CoA reductase (E.C. 1.1.1.34). Z. Naturforsch. 38c: 212–219.
TANZAWA, K., ENDO, A. 1979. Kinetic analysis of the reaction catalyzed by rat-liver 3-hydroxy-3-methylglutaryl-coenzyme A reductase using two specific inhibitors. Eur. J. Biochem. 98: 195–201.
ROGERS, D.H., RUDNEY, H. 1982. Modification of 3- hydroxy-3-methyl-glutaryl coenzyme A reductase immunoinhibition curves by substrates and inhibitors. Evidence for conformational changes leading to alterations in antigenicity. J. Biol. Chem. 251: 10650–10658.
CLELAND, W.W. 1963. The kinetics of enzyme-catalyzed reactions with two or more substrates. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim. Biophys. Acta 67: 188–196.
BACH, T.J. 1989. Zur Biosynthese und physiologischen Funktion der Mevalonsäure in Pflanzen. Habilitationsschrift, Fakultät für Bio und Geowissenschaften, Universität Karlsruhe, pp. 1–135.
FEYEREISEN, R., FARNSWORTH, D.E. 1987. Characterization and regulation of HMG-CoA reductase during a cycle of juvenile hormone synthesis. Mol. Cell. Endocrinol. 53: 227–238.
WOODWARD, H.D., ALLEN, J.M.C., LENNARZ, W.J. 1988. 3-Hydroxy-3-methylglutaryl coenzyme A reductase in the sea urchin, embryo is developmentally regulated. J. Biol. Chem. 263: 2513–2517.
GILL JR., J.F., RODWELL, V.W. 1983. Purification and properties of Pseudomonas HMG-CoA reductase. J. Lipid Res. 24: 1407.
GILL JR., J.F., BEACH, M.J., RODWELL, V.W. 1985. Mevalonate utilization in Pseudomonas sp. M. Purification and characterization of an inducible 3-hydroxy-3-methylglutaryl coenzyme A reductase. J. Biol. Chem. 260: 9393–9398.
LOWE, D.M., TUBBS, P.K. 1985. 3-Hydroxy-3-methylglutaryl-coenzyme A synthase from ox liver. Purification, molecular and catalytic properties. Biochem J. 221: 591–599.
MIZIORKO, H.M., BEHNKE, C.E. 1985. Active site-directed inhibition of 3-hydroxy-3-methylglutaryl coenzyme A synthase by 3-chloropropionyl coenzyme A. Biochemistry U.S.A. 24: 3174–3179.
MIZIORKO, H.M., BEHNKE, C.E. 1985. Amino acid seqence of an active site peptide of avian liver mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase. J. Biol. Chem. 260: 13513–13516.
GIL, G., GOLDSTEIN, J.L., SLAUGHTER, C.A., BROWN, M.S. 1986. Cytoplasmic 3-hydroxy-3-methylglutaryl coenzyme A synthase from the hamster: I. Isolation and sequencing of a full-length cDNA. J. Biol. Chem. 261: 3710–3716.
GIL, G., BROWN, M.S., GOLDSTEIN, J.L. 1986. Cytoplasmic 3-hydroxy-3-methylglutaryl coenzyme A synthase from the hamster: II. Isolation of the gene and characterization of the 5′ flanking region. J. Biol. Chem. 261: 3717–3724.
SMITH, J.R., OSBORNE, T.F., BROWN, M.S., GOLDSTEIN, J.L., GIL, G. 1988. Multiple sterol regulatory elements in the promoter for hamster 3-hydroxy-3-methylglutaryl-coenzyme A synthase. J. Biol. Chem. 263: 18480–18487.
DEQUIN, S., GLOECKLER, R., HERBERT, C.J., BOUTELET, F. 1988. Cloning, sequencing and analysis of the yeast S. uvarum ERGIO gene encoding acetoacetyl CoA thiolase. Curr. Genet. 13: 471–478.
OSBORNE, T.F., GIL, G., GOLDSTEIN, J.L., BROWN, M.S. 1988. Operator constitutive mutation of 3-hydroxy-3-methylglutaryl coenzyme A reductase promoter abolishes protein binding to sterol regulatory element. J. Biol. Chem. 263: 3380–3387.
DAWSON, P.A., HOFMANN, S.L., VAN DER WESTHUYZEN, D.R., SüDHOFF, T.C., BROWN, M.S., GOLDSTEIN, J.L. 1988. Sterol-dependent repression of low density lipoprotein receptor promoter mediated by 16-base pair sequence adjacent to binding site for transcription factor Spl. J. Biol. Chem. 263: 3372–3379.
GIL, G., SMITH, J.R., GOLDSTEIN, J.L., SLAUGHTER, C.A., ORTH, K., BROWN, M.S. 1988. Multiple genes encode nuclear factor 1-like proteins that bind to the promoter for 3-hydroxy-3-methylglutaryl-coentyme A reductase. Proc. Natl. Acad. Sci. 85: 8963–8967.
OSHIMA, K., URITANI, I. 1968. Enzymatic synthesis of a β-hydroxy-β-methylglutaric acid-derivative by a cell free system from sweet potato infected with black rot. J. Biochem. Tokyo 63: 617–625.
POTTY, V.H. 1969. Occurrence and properties of enzymes associated with mevalonic acid synthesis in the orange. J. Food Sci. 34: 231–234.
MESSNER, B., EGGERER, H., CORNFORTH, J.W., MALLABY, R. 1975. Substrate stereochemistry of the hxdroxymethyl-glutaryl-CoA lyase and methylglutaconyl-CoA hydratase reactions. Eur. J. Biochem. 53: 255–264.
NEMETHY, E.K., SKRUKRUD, C., PIAZZA, G.J., CALVIN, M. 1983. Terpenoid biosynthesis in Euphorbia latex. Biochim. Biophys. Acta 760: 343–349.
BACH, T.J., WEBER, T. 1989. Enzymic synthesis of mevalonic acid in plants. In: Biological Role of Plant Lipids. (P.A. Biacs, K. Gruiz, T. Kremmer, eds.), Akademiai Kiado, Budapest and Plenum Publishing Corporation, New York and London, pp. 279–282.
CLINKENBEARD, K.D., SUGIYAMA, T., MOSS, J., REED, W.D., LANE, M.D. 1973. Molecular and catalytic properties of cytosolic acetoacetyl coenzyme A thiolase from avian liver. J. Biol. Chem. 248: 2275–2284.
CLINKENBEARD, K.D., REED, W.D., MOONEY, R.A., LANE, M.D. 1975. Intracellular localisation of the 3-hydroxy-3-methylglutaryl coenzyme A cycle enzymes in liver: Separate cytoplasmic and mitochondrial 3-hydroxy-3-methylglutaryl coenzyme A generating systems for cholesterogenesis and ketogenesis. J. Biol. Chem. 250: 2108–3116.
CLINKENBEARD, K.D., SUGIYAMA, T., REED, W.D., LANE, M.D. 1975. Cytoplasmic 3-hydroxy-3-methylglutaryl coenzyme A synthase from liver: Purification, properties and role in cholesterol synthesis. J. Biol. Chem. 250: 3124–3135.
TOMODA, H., KUMAGAI, H., TANAKA, H., OMURA, S. 1987. F-244 specifically inhibits 3-hydroxy-3-methylglutaryl coenzyme A synthase. Biochim. Biophys. Acta 922: 351–356.
GREENSPAN, M. D., YUDKOVITZ, J.B., LO, C.Y.L., CHEN, J.S., ALBERTS, A.W., HUNT, V.M., CHANG, M.N., YANG, S.S., THOMPSON, K.L., CHIANG, Y.C.P., CHABALA, J.C., MONAGHAN, R.L., SCHWARTZ, R.L. 1987. Inhibition of hydroxymethylglutaryl-coenzyme A synthase by L-659,699. Proc. Natl. Acad. Sci. U.S.A. 84: 7488–7492.
BUCHER, N.L.R., OVERATH, P., LYNEN, F. 1960. β-Hydroxy-β-methylglutaryl coenzyme A reductase, cleavage and condensing enzymes in relation to cholesterol formation in rat liver. Biochim. Biophys. Acta 40: 491–501.
QUANT, P.A., TUBBS, P.K., BRAND, M.D. 1989. Glucagon increases mitochondrial 3-hydroxy-3-methylglutaryl-coenzyme A synthase activity in vivo by desuccinylating the enzyme. Biochem. Soc. Transact. 17: 147–148.
BALASUBRAMANIAM, S., GOLDSTEIN, J.L., BROWN, M.S. 1977. Regulation of cholesterol synthesis in rat adrenal gland through coordinate control of 3-hydroxy-3-methylglutaryl coenzyme A synthase and reductase activities. Proc. Natl. Acad. Sci. U.S.A. 74: 1421–1425.
CHIN, D.J., LUSKEY, K.L., ANDERSON, R.G.W., FAUST, J.R., GOLDSTEIN, J.L., BROWN, M.S. 1982. Appearance of crystalloid endoplasmic reticulum in compactin-resistant Chinese hamster cells with a 500-fold increase in 3-hydroxy-3-methylglutaryl coenzyme A reductase. Proc. Natl. Acad. Sci. U.S.A. 79: 1185–1189.
LUSKEY, K.L., FAUST, J.R., CHIN, D.J., BROWN, M.S., GOLDSTEIN, J.L. 1983. Amplification of the gene for 3-hydroxy-3-methylglutaryl coenzyme A reductase, but not for the 53-kDa protein, in UT-1 cells. J. Biol. Chem.258: 8462–8469.
LEONARD, S., ARBOGAST, D., GEYER, D., JONES, C., SINENSKY, M. 1986. Localization of the gene encoding 3-hydroxy-3-methylglutaryl-coenzyme A synthase to human chromosome 5. Proc. Natl. Acad. Sci. 83: 2187–2189.
HUMPHRIES, S.E., TATA, F., HENRY, I., BARICHARD, F., HOLM, M., JUNIEN, C., WILLIAMSON, R. 1985. The isolation, characterisation, and chromosomal assignment of the gene for human 3-hydroxy-3-methylglutaryl coenzyme A reductase, (HMG-CoA reductase). Hum. Genet. 71: 254–258.
LINDGREN, V., LUSKEY, K.L., RUSSELL, D.W., FRANCKE, U. 1985. Human genes involved in cholesterol metabolism: Chromosomal map** of the loci for the low density lipoprotein receptor and 3-hydroxy-3-methylglutaryl-coenzyme A reductase with cDNA probes. Proc. Natl. Acad. Sci. 82: 8567–8571.
KORNBLATT, J., RUDNEY, H. 1971. Two forms of acetoacetyl coenzyme A thiolase in yeast: I. Separation and properties. J. Biol. Chem.246: 4417–4423.
KORNBLäTT, J., RUDNEY, H. 1971. Two forms of acetoacetyl coenzyme A thiolase in yeast: II. Intracellular location and relationship to growth. J. Biol. Chem.246: 4424–4430.
TROCHA, P.J., SPRINSON, D.B. 1976. Location and regulation of early enzymes of sterol biosynthesis in yeast. Arch. Blochem. Biophys. 174: 45–51.
SERVOUSE, M., KARST, F. 1986. Regulation of early enzymes of ergosterol biosynthesis in Saccharomyces cerevisiae. Biochem. J. 240: 541–547.
KAWAGUCHI, A. 1970. Control of ergosterol biosynthesis in yeast: Existence of lipid inhibitors. J. BioChem.67: 219–227.
BOLL, M., LOWELL, M., STILL, J., BERNDT, J. 1975. Sterol biosynthesis in yeast. Eur. J. BioChem.54: 435–444.
QUAIN, D.E., HASLAM, J.M. 1979. The effects of catabolite derepression on the accumulation of steryl esters and the activity of β-hydroxy-β-methylglutaryl-CoA reductase in Saccharomyces cerevisiae. J. Gen. MicroBiol. 11: 343–351.
BERG, D., DRABER, W., VON HUGO, H., HUMMEL, W., MAYER, D. 1981. The effect of clotrimazole and triadimefon on 3-hydroxy-3-methyl-glutaryl-CoA reductase-[EC 1.1.1.34]-activity in Saccharomyces cerevisiae. Z. Naturforsch. 36c: 798–803.
DéQUIN, S., BOUTELET, F., SERVOUSE, M., KARST, F. 1988. Effect of acetoacetyl CoA thiolase amplification on sterol synthesis in the yeasts S. cerevisiae and S. uvarum. Biotechnol. Lett. 10: 457–462.
KRAMER, P.R., MIZIORKO, H.M. 1983. 3-Hydroxy-3-methylglutaryl-CoA lyase: Catalysis of acetyl coenzyme A enolization. Biochemistry USA 22: 2353–2357.
STEGINK, L.D., COON, M.J. 1968. Stereospecificity and other properties of highly purified β-hydroxy-β-methylglutaryl coenzyme A cleavage enzyme from bovine liver. J. Biol. Chem.243: 5272–5279.
BACHHAWAT, B. K., ROBINSON, W.G., COON, M.J. 1955. The enzymatic cleavage of β-hydroxy-β-methylglutaryl-coenzyme A to acetoacetate and acetyl coenzyme A. J. Biol. Chem.216: 727–736.
HIGGINS, M.J.P., KORNBLATT, J.A., RUDNEY, H. 1972. Acyl-CoA ligases. The Enzymes, Vol. VII (P.D. Boyer, ed.), Academic Press, New York, pp. 407–434.
POPJàk, G. 1971. Specificity of enzymes of sterol biosynthesis. Harvey Lect. 65: 127–156.
LANDAU, B.R., BRUNENGRäBER, H. 1985. Shunt pathway of. mevalonate metabolism. Meth. Enzymol. 110: 100–114.
NES, W.D., CAMPBELL, B.C., STAFFORD, A.E., HADDON, W.F., BENSON, M. 1982. Metabolism of mevalonic acid to long chain fatty alcohols in an insect. Biochem. Biophys. Res. Commun. 108: 1258–1263.
NES, W.D., BACH, T.J. 1985. Evidence for a mevalonate shunt in a tracheophyte. Proc. Roy. Soc. Lond. B 225: 425–444.
ALLEN, K.G., BANTHORPE, D.V., CHARLWOOD, B.V., EKUNDAYO, O., MANN, J. 1976. Metabolic pools associated with monoterpene biosynthesis in higher plant. Phytochemistry 15: 101–107.
SINGH, S.S., NEE, T.Y., POLLARD, M.R. 1986. Acetate and mevalonate labeling studies with develo** Cuphea lutea seeds. Lipids 21: 143–149.
GROENEVELD, H.W., ELINGS, J.C., JORGE BLANCO, M.S., BRAMWELL, D. 1989. Quantitative aspects of triacylglycerol metabolism and sterol synthesis from 14C-acetate in etiolated seedlings of Euphorbia lambii. Physiol. Plant. 75: 227–232.
SAUVAIRE, Y., TAL, B., HEUPEL, R.C., ENGLAND, R., HANNERS, P.K., NES, W.D., MUDD, J.B. 1987. A comparison of sterol and long chain fatty alcohol biosynthesis in Sorghum bicolor. In: The Metabolism, Structure, and Function of Plant Lipids. (P.K. Stumpf, J.B. Mudd, W.D. Nes, eds.), Plenum Press, New York — London, pp. 107–110.
BAISTED, D.J., NES, W.R. 1963. The relative efficacy of mevalonic and dimethylacrylic acids as isopentenoid precursors in germinating seeds of Pisum sativum. J. Biol. Chem.238: 1947–1952.
KANNANGARA, C.G., JENSEN, C.G. 1975. Biotin carboxyl carrier protein in barley chloroplast membranes. Eur. J. BioChem.54: 25–30.
NIKOLAU, B.J., WURTELE, E.S., STUMPF, P.K. 1984. Subcellular distribution of acetyl-coenzyme A carboxylase in mesophyll cells of barley and sorghum leaves. Arch. Biochem. Biophys. 235: 555–561.
NIKOLAU, B. J., WURTELE, E.S., STUMPF, P.K. 1985. Use of streptavidin to detect biotin-containing proteins in plants. Anal. BioChem.149: 448–453.
HOFFMAN, N.E., PICHERSKY, E., CASHMORE, A.R. 1987. A tomato cDNA encoding a biotin-binding protein. Nucl. Acids Res. 15: 3928.
ITO, M., FUKUI, T., SAITO, T., TOMITA, K. 1987. Inhibition of acetoacetyl-CoA synthetase from rat liver by fatty acyl-CoAs. Biochim. Biophys. Acta 922: 287–293.
WONG, G.A., BERGSTROM, J.D., EDMOND, J. 1987. Acetoacetyl-CoA ligase activity in the isolated rat hepatocyte effects of 25-hxdroxycholesterol and high density lipoprotein. Biosci. Rep. 7: 217–224.
SALAM, W.H., CAGEN, L.M., HEIMBERG, M. 1988. Regulation of hepatic cholesterol biosynthesis by fatty acids: Effects of feeding olive oil on cytoplasmic acetoacetyl-coenzyme A thiolase, β-hydroxy-β-methylglutaryl-CoA synthase, and acetoacetyl-coenzyme A ligase. Biochem. Biophys. Res. Commun. 153: 422–427.
HARWOOD, J.L. 1987. Lipid metabolism. In: The Lipid Handbook. (F.D. Gunstone, J.L. Harwood, F.B. Padley, eds.), Chapman and Hall, London — New York, pp. 485–503.
BEHRENDS, W., ENGELAND, K., KINDL, H. 1988. Characterization of two forms of the multifunctional protein acting in fatty acid β-oxidation. Arch. Biochem. Biophys. 263: 161–169.
ERNST-FONBERG, M.L. 1986. An NADH-dependent acetoacetyl-CoA reductase from Euglena gracilis. Purification and characterization, including inhibition by acyl carrier protein. Plant Physiol. 82: 978–984.
KURIHARA, T., UEDA, M., TANAKA, A. 1988. Occurrence and possible roles of acetoacetyl-CoA thiolase and 3-ketoacyl-CoA thiolase in peroxisomes of an n-alkane-grown yeast, Candida tropicalis. FEBS Lett. 229: 215–218.
ENDO, T., HAMAGUCHI, N., HASHIMOTO, T., YAMADA, Y. 1988. Non-enzymatic synthesis of hygrine from acetoacetic acid and from acetonedicarboxylic acid. FEBS Lett. 234: 86–90.
HAUGHAN, P.A., LENTON, J.R., GOAD, L.J. 1987. Inhibition of growth of celery cells by paclobutrazol and its reversal by added sterols. In: The Metabolism, Structure, and Function of Plant Lipids. (P.K. Stumpf, J.B. Mudd, W.D. Nes, eds.), Plenum Press, New York — London, pp. 91–93).
GRUNDY, S.M. 1988. HMG-CoA reductase inhibitors for treatment of hypercholesterolemia. N. Engl. s J. Med. 319: 24–33.
BACH, T.J., LICHTENTHALER, H.K. 1987. Plant growth regulation by mevinolin and other sterol biosynthesis inhibitors. In: Ecology and Metabolism of Plant Lipids. (G. Fuller and W.D. Nes, eds.), Am. Chem. Soc. Symposium Series 325, American Chemical Society, Washington, pp. 109–139.
BACH, T.J., LICHTENTHALER, H.K. 1982 Mevinolin: A highly specific inhibitor of microsomal 3-hydroxy-3-methylglutaryl-coenzyme A reductase of radish plants. Z. Naturforsch. 37c: 46–50.
BACH, T.J., LICHTENTHALER, H.K. 1982. Inhibition by mevinolin of mevalonate formation and plant root elongation. Naturwissenschaften 69: 242.
BACH, T.J., LICHTENTHALER, H.K. 1983. Inhibition by mevinolin of plant growth, sterol formation and pigment accumulation. Physiol. Plant. 59: 50–60.
BACH, T.J. 1985. Selected natural and synthetic enzyme inhibitors of sterol biosynthesis as molecular probes for in vivo studies concerning the regulation of plant growth. Plant Sci. 39: 183–187.
HATA, S., TAKAGISHI, H., EGAWA, Y., OTA, Y. 1986. Effects of compactin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, on the growth of alfalfa (Medicago sativa) seedlings and the rhizogenes of pepper (Capsicum annuum) expiants. Plant Growth Regul. 4: 335–346.
SCHINDLER, S., BACH, T.J., LICHTENTHALER, H.K. 1985. Differential inhibition by mevinolin of prenyllipid accumulation in radish seedlings. Z. Naturforsch. 40c: 208–214.
PENNOCK, J.F., THRELFALL, D.R. 1983. Biosynthesis of ubiquinones and related compounds. In: Biosynthesis of Isoprenoid Compounds. (J.W. Porter, S.L. Spurgeon, eds.), Vol. 2, J. Wiley, New York, pp. 291–303.
CAMARA, B., BARDAT, F., SEYE, A., D’harlingue, A., Monéger, R. 1982. Terpenoid metabolism in plastids. Localization of α-tocopherol synthesis in Capsicum chromoplasts. Plant Physiol. 70: 1562–1563.
SCHULTZ, G., SOLL, J., FIEDLER, E., SCHULZE-SIEBERT, D. 1985. Synthesis of prenylquinones in chloroplasts. Physiol. Plant. 64: 123–129.
SOLL, J., SCHULTZ, G., JOYARD, J., DOUCE, R., BLOCK, M.A. 1985. Localization and synthesis of prenylquinones in isolated outer and inner envelope membranes from spinach chloroplasts. Arch. Biochem. Biophys. 238: 290–299.
SCHINDLER, S., LICHTENTHALER, H.K. 1982. Distribution and levels of ubiquinone homologues in higher plants. In: Biochemistry and Metabolism of Plant Lipids. (J.F.G.M. Wintermans, P.J.C. Kuiper, eds.), Elsevier-North Holland Biomedical Press, Amsterdam, pp. 545–548.
BACH, T.J., NES, W.D. 1984. Evidence for a mevalonate shunt pathway in wheat. In: Structurer Function and Metabolism of Plant Lipids. (P.A. Siegenthaler, W. Eichenberger, eds.), Elsevier Science Publishers BV, Amsterdam, pp. 217–220.
LICHTENTHALER, H.K., BACH, T.J., WELLBURN, A. 1982. Cytoplasmic and plastidic isoprenoid compounds of oat seedlings and their distinct labelling from 14C-mevalonate. In: Biochemistry and Metabolism of Plant Lipids. (J.F.G.M. Wintermans, P.J.K. Kuiper, eds.), Elsevier, Amsterdam, pp. 489–500.
SCHULZE-SIEBERT, D., SCHULTZ, G. 1987. Full autonomy in isoprenoid synthesis in spinach chloroplasts. Plant Physiol. BioChem.25: 145–153.
HATA, S., TAKAGISHI, H., KOUCHI, H. 1986. Variation in the content and composition of sterols in alfalfa seedlings treated with compactin (ML-236B) and mevalonic acid. Plant Cell Physiol. 28: 709–714.
HATA, S., SHIRATA, K., TAKAGISHI, H., KOUCHI, H. 1987. Accumulation of rare phytosterols in plant cells on treatment with metabolic inhibitors and mevalonic acid. Plant Cell Physiol. 28: 715–722.
FAUST, J.R., GOLDSTEIN, J.L., BROWN, M.S. 1979. Synthesis of ubiquinone and cholesterol in human fibroblasts: Regulation of a branched pathway. Arch. Biochem. Biophys. 192: 86–89,
JAMES, M.J., KANDUTSCH, A.A. 1979. Inter-relationships between dolichol and sterol synthesis in mammalian cell cultures. J. Biol. Chem.254: 8442–8446.
KELLER, G. A., PAZIRANDEH, M., KRISANS, S. 1986. 3- Hydroxy-3-methyl-glutaryl-coenzyme A reductase localization in rat liver peroxisomes and microsomes of control and cholestyramine-treated animals: quantitative biochemical and immunoelectron microscopical analyses. J. Cell Biol. 103: 875–886.
FAUST, J.R., BROWN, M.S., GOLDSTEIN, J.L. 1980. Synthesis of A2-isopentenyl tRNA from mevalonate in cultured human fibroblasts. J. Biol Chem.255: 6546–6548.
KENDE, H. 1965. Kinetin-like factors in the root exudate of sunflowers. Proc. Natl. Acad. Sci. U.S.A. 53: 1302–1307.
WEISS, C., VAADIA, Y. 1965. Kinetin-like activity in root apices of sunflower plants. Life Sci. 4: 1323–1326.
BUSCHMANN, C., LICHTENTHALER, H.K. 1982: The effects of cytokinins on growth and pigment accumulation of radish seedlings (Raphanus sativus L.) grown in the dark and at different light quanta fluence rates. Photochem. PhotoBiol. 35: 217–221.
BITTNER, A., BUSCHMANN, C. 1983 Uptake and translocation of K+, Ca2+ and Mg2 + by seedlings of Raphanus sativus L. treated with kinetin. Z. Pflanzenphysiol. 109: 191–189.
DOLL, M., SCHINDLER, S., LICHTENTHALER, H.K., BACH, T.J. 1984. Differential inhibition by mevinolin of prenyllipid accumulation in cell suspension cultures of Silybum marianum L. In: Structure, Function and Metabolism of Plant Lipids. (P.A. Siegenthaler and W. Eichenberger, eds.), Elsevier Science Publishers BV, Amsterdam, pp. 277–280.
SCHINDLER, S. 1984. Verbreitung und Konzentration von Ubichinon-Homologen in Pflanzen. Karls. Beitr. Pflanzenphysiol. (Karlsr. Contrib. Plant Physiol., ISSN 0173–3133) 12: 1–240.
RYDER, N.S., GOAD, L.J. 1980: The effect of the 3-hydroxy-3-methylglutaryl CoA reductase inhibitor ML-236B on phytosterol synthesis in Acer pseudoplantanus tissue culture. Biochim. Biophys. Acta 619: 424–427.
HASHIZUME, T., MATSUBARA, S., ENDO, A. 1983. Compactin (ML-236B) as a new growth inhibitor of plant callus. Agric. Biol. Chem.47: 1401–1403.
MCCHESNEY, J.D. 1970. Stimulation of in vitro growth of plant tissues by D,L-mevalonic acid. Can. J. Bot. 48: 2357–2359.
MURAI, N., ARMSTRONG, D.J., SKOOG, F. 1975: Incorporation of mevalonic acid into ribosylzeatin in tobacco callus ribonucleic acid preparations. Plant Physiol. 55: 853–858.
CHEN, T.H., SHIAO, M.S., HSIEH, W.L. 1987. Inhibition of somatic embryogenesis in cultured carrot cells by mevinolin. Bot. Bull. Academia Sinica 28: 211–217.
FAUST, J.R., LUSKEY, K.L., CHIN, D.J., GOLDSTEIN, J.L., BROWN, M.S. 1982. Regulation of synthesis and degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase by low density lipoprotein and 25-hydroxycholesterol in UT-1 cells. Proc. Natl. Acad. Sci. U.S.A. 79: 5205–5209.
LUSKEY, K.L., CHIN, D.J., MACDONALD, R.J. K.L., GOLDSTEIN, J.L., BROWN, M.S. 1982. Identification of a cholesterol-regulated 53,000-dalton cytosolic protein in UT-1 cells and cloning of its cDNA. Proc. Natl. Acad. Sci. U.S.A. 79: 6210–6214.
HARDEMAN, E.C., JENKE, H.S., SIMONI, R.D. 1983. Overproduction of a M, 92,000 protomer of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in compactin-resistant C100 cells. Proc. Natl. Acad. Sci. U.S.A. 80: 1516–520.
HARDEMAN, E.C., ENDO, A., SIMONI, R.D. 1984. Effects of compactin on the levels of 3-hydroxy-3-methylglutaryl coenzyme A reductase in compactin-resistant C100 and wild-type cells. Arch. Biochem. Biophys. 232: 549–561.
MASUDA, A., KUWANO, M., SHIMADA, T. 1983. Ultrastructural changes during the enhancement of cellular 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase in a Chinese hamster cell mutant resistant to compactin (ML 236B). Cell Struct. Function 8: 309–312.
ORCI, L., BROWN, M.S., GOLDSTEIN, J.L., GARCIA-SEGURA, L.M., ANDERSON, R.G.W. 1984. Increase in membrane cholesterol: a possible trigger for degradation of HMG CoA reductase and crystalloid endoplasmic reticulum in UT-1 cells. Cell 36: 835–845.
HASUMI, K., YAMADA, A., SHIMIZU, Y., ENDO, A. 1987. Overaccumulation of 3-hydroxy-3-methylglutaryl-coenzyme-A reductase in a compactin (ML-236B)-resistant mouse cell line with defects in the regulation of its activity. Eur. J. BioChem.164: 547–552.
GOODWIN, T.W. 1958. Incorporation of 14CO2, [2-14C]acetate and [2-14C] mevalonic acid into β-carotene in etiolated maize seedlings. Biochem. J. 68: 26–27.
GOODWIN, T.W. 1958. Studies in carotenogenesis. 25. The incorporation of 14CO2, [2-14C]acetate and [2-14C]mevalonic acid into β-carotene by illuminated etiolated maize seedlings. Biochem. J. 70: 612–617.
GOODWIN, T.W. 1965. The biosynthesis of carotenoids. In: Biosynthetic Pathways in Plants. (J.B. Pridham, T. Swain, eds.), Academic Press, London, pp. 37–55.
ROGERS, L.J., SHAH, S.P.J., GOODWIN, T.W. 1966. Compartmentation of terpenoid biosynthesis in green plants. Biochem. J. 104: 395–405.
SCHULTZ, G., HUCHZERMEYER, Y., REUPKE, B., BICKEL, H. 1976. On the intra-cellular site of biosynthesis of α-tocopherol in Hordeum vulgare. Phytochemistry, 15: 1253–1255.
GRUMBACH, K.H., FORN, B. 1980. Chloroplast autonomy in acetyl-coenzyme A formation and terpenoid biosynthesis. Z. Naturforsch. 35c: 645–648.
GRAY, J.C., KEKWICK, R.G.O. 1973. Mevalonate kinase in green leaves and etiolated cotyledons of the french bean Phaseolus vulgaris. Biochem. J. 133: 335–347.
HILL, H.W., ROGERS, L.J. 1974. Mevalonate activating enzymes and phosphatases in higher plants. Phytochemistry 13: 763–777.
KREUZ, K., KLEINIG, H. 1981. On the compartmentation of isopentenyl diphosphate synthesis and utilization in plant cells. Planta 153, 578–581.
LüTKE-BRINKHAUS, F., LIEDVOGEL, B., KLEINIG, H. 1984. On the biosynthesis of ubiquinones in plant mitochondria. Eur. J. BioChem.141: 537–541.
LIEDVOGEL, B. 1986. Acetyl coenzyme A and isopentenyl pyrophosphate as lipid precursors in plant cells -biosynthesis and compartmentation. J. Plant Physiol. 124: 211–222.
KLEINIG, H. 1987. Carotenoid biosynthesis and carotenogenic enzymes in plants. In: The Metabolism, Structure, and Function of Plant Lipids. (P.K. Stumpf, J.B. Mudd, W.D. Nes, eds.), Plenum Press, New York — London, pp. 37–43.
EMMANUEL, B., ROBBLEE, A.R. 1984. Cholesterogenesis from propionate: facts and speculations. Int. J. BioChem.16: 907–911.
DAVIS, A.R. 1978. Participation of propionate in cholesterol biosynthesis in rat liver. Steroids 31: 593–600.
HALARNKAR, P.P., WAKAYAMA, E.J., BLOMQUIST, G.J. 1988. Metabolism of propionate to 3-hydroxypropionate and acetate in the Lima bean Phaseolus limensis. Phytochemistry 27: 997–999.
SCHULTZ, G., SCHULZE-SIEBERT, D. 1989. Chloroplast isoprenoid synthesis in spinach and barley: direct intermediate supply by photosynthetic carbon fixation. In: Biological Role of Plant Lipids. (P.A. Biacs, K. Gruiz, T. Kremmer, eds.), Akademiai Kiado, Budapest, and Plenum Publishing Corporation, New York — London, pp. 313–319.
STITT, M., AP REES, T. 1979. Capacities of pea chloroplasts to catalyse the oxidative pentose pathway and glycolysis. Phytochemistry 18: 1905–1911.
MCHALE, N. A., HANSON, K.R., ZELITCH, I. 1988. A nuclear mutation in Nicotiana sylvestris causing a thiamine-reversible defect in synthesis of chlorplast pigments. Plant Physiol. 88: 930–935.
CURRY, K.J. 1987. Initiation of terpenoid synthesis in osmophores of Stanhopea anfracta (Orchidaceae): a cytochemical study. Amer. J. Bot. 74: 1332–1338.
KELLER, G.A., BARTON, M.C., SHAPIRO, D.J., SINGER, S.J. 1985. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase is present in peroxisomes in normal rat liver cells. Proc. Natl. Acad. Sci. U.S.A. 82: 770–774.
SHAPIRO, D.J., GOULD, L., MARTIN, G., WILLIAMS, D., WOLSKI, R. 1986. Organization and regulated expression of rat liver HMG-CoA reductase genes. J. Lipid Res. 27: 567–568.
REYNOLDS, G. A., GOLDSTEIN, J.L., BROWN, M.S. 1985. Multiple mRNAs for 3-hydroxy-3-methylglutaryl coenzyme A reductase determined by multiple transcription initiation sites and intron splicing sites in the 5′-untranslated region. J. Biol. Chem.260: 10369–10377.
GIL, G., FAUST, J.R., CHIN, D.J., GOLDSTEIN, J.L., BROWN, M.S. 1985. Membrane-bound domain of HMG CoA reductase is required for sterol-enhanced degradation of the enzyme. Cell 41: 249–258.
LUSKEY, K.L. 1987. Conservation of promoter sequence but not complex intron splicing pattern in human and hamster genes for 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mol. Cell. Biol. 7: 1881–1893.
THOMPSON, S.L., BURROWS, R., LAUB, R.J., KRISANS, S.K. 1987. Cholesterol synthesis in rat liver peroxisomes. Conversion of mevalonic acid to cholesterol. J. Biol. Chem.262: 17420–17425.
HASLAM, J.M., BIRCH, D.J., CRABBE, T.B. 1986. The control of 3-hydroxy-3-methylglutaryl-CoA reductase activity in Saccharomyces cerevisiae. J. Lipid Res. 27: 563.
BOLL, M. 1983. 3-Hydroxy-3-methylglutaryl coenzyme A reductase in yeast and other microorganismes. In: 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase. (J.R. Sabine, ed.), CRC Series in Enzyme Biology, CRC Press, Inc., Boca Raton, Florida, pp. 39–53.
BASSON, M.E., THORSNESS, M., RINE, J. 1986. Saccharomyces cerevisiae contains two functional genes encoding 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Broc. Natl. Acad. Sci. U.S.A. 83: 5563–5567.
CAELLES, C., FERRER, A., BALCELLS, L., HEGARDT, F.G., BORONAT, A. 1989. Isolation and structural characterization of a cDNA encoding Arabidopsis thaliana 3-hydroxy-3-methylglutaryl coenzyme A reductase. Plant. Mol. Biol, (in press).
LEARNED, R.M., FINK, G.R. 1989. 3-Hydroxy-3- methylglutaryl-coenzyme A reductase from Arabidops. thaliana is structurally distinct from the yeast al animal enzymes. Proc. Natl. Acad. Sci. U.S.A. 86: 2779–2783.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1990 Plenum Press, New York
About this chapter
Cite this chapter
Bach, T.J., Weber, T., Motel, A. (1990). Some Properties of Enzymes Involved in the Biosynthesis and Metabolism of 3-Hydroxy-3-Methylglutaryl-CoA in Plants. In: Towers, G.H.N., Stafford, H.A. (eds) Biochemistry of the Mevalonic Acid Pathway to Terpenoids. Recent Advances in Phytochemistry, vol 24. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-8789-3_1
Download citation
DOI: https://doi.org/10.1007/978-1-4684-8789-3_1
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4684-8791-6
Online ISBN: 978-1-4684-8789-3
eBook Packages: Springer Book Archive