Abstract
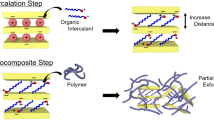
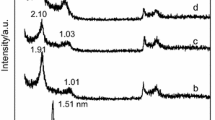
Intercalation compounds generally consist of porous host structures which can ingest a variety of guest species into the pore spaces with little or no distortion of the host structure itself. 1–3 The porous regions within the host may exhibit full three dimensional connectivity as in zeolites4 or alkali tungsten bronzes5, completely constrained one dimensional character as in polyacetylene6 or partially constrained two dimensional regions as in graphite intercalation compounds,7 intercalated layer dichalcogenides,8 and clay intercalation compounds. These latter solids are members of a large class of materials, the layered intercalation compounds, which has been heavily investigated during the past decade. This class of intercalation compound has been interesting primarily because it exhibits not only unusual physical properties which may be of technological importance but also because it serves as an arena in which to study novel physical phenomena in reduced, i.e. 2, dimensions.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
S.A. Solin and H. Zabel, The physics of ternary graphite intercalation compounds, Adv. Phys. 37:87 (1988).
S.A. Solin, The nature and structural properties of graphite intercalation compounds Adv. Chem. Phys. 49:455 (1985).
S.A. Solin, Alumino-silicate clays and clay intercalation compounds, in: “Intercalation in Layered Materials,” M.S. Dresselhaus, ed. Plenum, New York (1986), p. 145.
D.W. Breck, “Zeolite Molecular Sieves. Structure, Chemistry and Use,” Wiley Interscience, New York (1974).
EJ. Flynn, S.A. Solin and H.R. Shanks, Raman active soft modes and phase transitions in sodium tungsten bronzes, Solid State Commun. 25:743 (1978), and references therein.
D. Djurado, J.E. fischer, P.A. Heiney, J. Ma, N. Coustel and P. Bernier, Staging in potassium doped polyacetylene: in situ X-ray diffraction, Synthetic Metals 34:683 (1989).
See refs. 1 and 2 and references therein.
W.Y. Liang, Electronic properties of transition metal dichalcogenides and their intercalation complexes, in: “Intercalation in Layered Materials,” M.S. Dresselhaus, ed. Plenum, New York (1986), p.31.
R.E. Grimm, “Clay Mineralogy,” McGraw Hill, New York (1968).
G.W. Brindly and G. Brown, eds., “Crystal Structures of Clay Minerals and their Identification,” Minerological Society, London (1980).
U.V.A. Drits and C. Tchoubar, “X-Ray Diffraction by Disordered Lamellar Structures,” Springer-Verlag, Berlin (1990).
T. J. Pinnavaia, Intercalated Clay Catalysts, Science 220:365 (1983).
S.N. Hoda and G.H. Beal, Alkaline earth mica glass-ceramics, Adv. in Ceramics 4:287 (1982).
S.W. Bailey, Structural studies of clay minerals during 1981–1985, in “Proceedings of the International Clay Conference, Denver, 1985,” L.g. Schultz, H. van Olphen and F.A. Mumpton, eds., Clay Minerals Society, Bloomington (1987).
M. Lipsicas, R.H. Raythatha, T.J. Pinnavaia, I.D. Johnson, R.F. Geise, P.M. Costanzo, and J.L. Robert, Silicon and aluminum site distributions in 2:1 layer silicate clays, Nature 309:604 (1984).
S.W. Baily, Cation ordering and psuedosymmetry in layer silicates, Am. Minerology 60:175 (1975).
C. de la Calle and H. Suquet, Vermiculite, Rev. in Minerology 19:455 (1988).
B. Velde, “Clays and Clay Minerals in Natural and Synthetic Systems,” Elsevier, Amsterdam (1977).
R.M. Hazen and D.R. Wones, The effect of cation substitutions on the physical properties of trioctahedral micas, Am. Mineral. 57:103 (1972).
D.M.C. MacEwan, Interlamellar reactions of clays and other substances, Clays and Clay Minerals 9:431 (1960).
G.W. Brindley and M.E. Harward, Swelling properties of synthetic smectites in relation to lattice substitutions, in “Clays and Clay Minerals, Proc. 13th Natl. Conf., Mfadison, Wisconsin, 1964,” W.F. Bradley and S.W. Bailey, eds., Pergamon Press, New York (1965).
N. Wada, D.R. Hines and S.P. Ahrenkiel, X-ray diffraction studies of hydration transitions in Na vermiculite, Phys. Rev. B41:12895 (1990).
W. Shockley, “Electrons and Holes in Semiconductors,” Van Nostrand, New York (1950).
B.R. York, S.A. Solin, N. Wada, R. Raythatha, I.D. Johnson and T.J. Pinnavaia, Substrate rigidity effects in mixed layered solids, Solid State Commun., 54:475 (1985).
H.P. Klug and L.E. Alexander, “X-Ray Diffraction Procedure for Polycrystalline and Amorphous Materials,” Wiley, New York (1974).
A. Guinier, “X-Ray Diffraction,” Freeman, San Francisco (1963).
R.W.G. Wycoff, “Crystal Structures, Vol. I,” Oxford University Press, London (1962), p26.
C. de la Calle, H. Suquet and C.H. Pons, Layer stacking order in a 14.30 Å Mg vermiculite, Clays and Clay Minerals, in press.
J.F. Alcover and L. Gatineau, Structure del l’espace interlamellaire de la vermiculite Mg bicouche, Clay Minerals 15:25 (1980).
H. Kim, J. Wei, S. Lee, P. Zhou, T.J. Pinnavaia, S.D. Mahanti and S.A. Solin, Layer rigidity and collective effects in pillared lamellar solids, Phys.Rev. Lett. 60:2168 (1988).
S. Lee and S.A. Solin, X-Ray Study of the intercalant distribution in mixed alkyl ammonium pillared clay, Phys. Rev. 43:2012 (1991).
S.B. Hendricks and E. Teller, X-ray interference in partially ordered layer lattices, J. Chem. Phys. 10:147 (1942).
D.M.C. MacEwan, Fourier transform method s for studying X-ray scattering from lamellar systems. I. A direct method for analyzing interstratified mixtures, Kolloidzeitschrift 149:96 (1956).
S.A. Solin, Novel properties of inercalated layered solids: from graphite to sheet silicates, J. Molec. Catalysis 27:293 (1984).
See ref. 3 and references therein.
S. Lee, H. Miyazaki, S.D. Mahanti and S.A. Solin, Composition-driven c-axis expansion of intercalated layered solids: Id non-Vegard’s-law behavior in a 2d solid solution, Phys. Rev. Lett. 62:3066 (1989).
S.A. Solin, H. Miyazaki, S. Lee and S.D. Mahanti, Static relaxations of puckering distortions in intercalated layered solids, J. Non-Cryst. Solids 131:1213 (1991).
M.F. Thorpe, Layer rigidity and spacing in intercalation compounds, Phys. Rev. B30:10370 (1989).
L. Vegard and H. Dale, Unterschungen ueber mischkristalle und legierungen, Zeits. Dristallogr. 67:148 (1928).
J.R. Dann, D.C. Dann and R.R. Haering, Elastic energy and staging in intercalation compounds, Solid State Commun. 42:179 (1982).
J.E. Fischer and H.J. Kim, Elastic effects in intercalation compounds: comparison of lithium in graphite and TiS2, Phys. Rev. B35:3295 (1987).
S.A. Safran, Stage ordering in intercalation compounds, in “Solid State Physics, Vol. 40,” D. Turnbull and H. Ehrenreich, eds., Academic Press, New York (1987).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1993 Springer Science+Business Media New York
About this chapter
Cite this chapter
Solin, S.A. (1993). The Structure, Morphology and Layer Rigidity of Clay Intercalation Compounds. In: Bernier, P., Fischer, J.E., Roth, S., Solin, S.A. (eds) Chemical Physics of Intercalation II. NATO ASI Series, vol 305. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-2850-0_8
Download citation
DOI: https://doi.org/10.1007/978-1-4615-2850-0_8
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4613-6234-0
Online ISBN: 978-1-4615-2850-0
eBook Packages: Springer Book Archive