Abstract
Adenosine deaminase (ADA) is one of the important enzymes acting in the metabolism of nucleic acid components. Lack of ADA has been shown to be associated with inherited severe combined immunodeficiency (SCID) and acquired immunodeficiency syndrome (AIDS). A marked increase in ADA has also been implicated in a number of other clinical conditions including, hereditary haemolytic anaemia and leukemias. Therefore, the great importance of research on inhibition, activation of ADA is required.
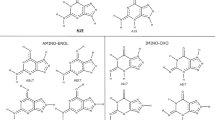
Here, the inhibition and activation of ADA by derivatives of acyclic adenine nucleoside (Compounds I, II, C — IX) substituted at the ninth adenine position are studied and denaturation of ADA by sodium n — dodecyl sulphate (SDS) and dodecyl trimethylammonium bromide have been also considered kinetically and thermodynamically at phosphate buffer, pH 7.5 in various temperatures.
The type of inhibition was analysed thermodynamically, using melting point (Tm) as a sensitive point for conformational change. The activation and deactivation of ADA were also investigated by pK method to obtain the amino acids which are interacted with inhibitors and activator. The glutamic, aspartic acids and a little histidine was involved to C — IX for activation. The deactivation was occured by change on histidine only.
The denaturation of ADA was also studied to obtain more information on the structure of the enzyme. The interaction of SDS caused the folding, whereas DTAB caused the unfolding which is corresponded to activation and deactivation for ADA respectively. The folding of ADA by SDS induced the minimum solubility conformed at lower temperature which means the greater apolar interactions in the interior phase result in a lower value for TH (temperature of minimum solubility), whereas it is inconsistent to unfolding state which is induced by ADA — DTAB complexes that is occured in a higher value for TH.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Worthington, C. (1988) Worthington Manual, enzymes, related biochemicals, pp.11, Worthington Biochemical Corporation Freehold, New Jersey.
Martin, D.W. and Gelfand, E. W. (1981) Ann. Rev. Biochem. 50: 845–877.
Sharff, A.J., Wilson, D.K., Chang, Z. and Quiocho, F.A. (1992) J. Mol. Biol. 226: 917–921.
Daddona, P.E. and Kelley, W.N. (1978) J. Biol. Chem. 253: 4617.
Giblett, E.R., Anderson, J.E., Cohen, F., Pollara, B. and Meuwissen, H. J. (1972) Lancet 2: 1067–1069.
Coleman, M.S., Donofrio, J., Hutton, J.J. and Hahn, L. (1978) J. Biol. Chem. 253: 1619–1626.
Verma, I.M. (1990) Scientific American 263, 34–41.
Cowan, M.J., Brady, R.O. and widder, K.J. (1986) Proc. Natl. Acad. Sci. U.S.A., 83: 1089–1091.
Can, T.E., Daddona, P.E. and Mitchel, B.S. (1987) Blood 69: 1376–1380.
Hakimelahi, G.H., Khalafi — Nezhad, A. and Mohanazadeh, F. (1990) Int. J. Chemistry 1: 9.
Hakimelahi, G.H., Zarrinehzad, M., Jarrahpour, A.A. and Sharghi, H., (1987) Helvetica Chimica Acta 70: 219.
Schaeffer, H.J. (1983) In: Nucleoside, nucleotides and their biological application (Rideout, J.L., Henry, D.W. and Beacham L.M. eds.) Academic press, New York.
Farzami, B., Moosavi — Movahedi, A.A. and Naderi, G.A. (1994) Int. J. Biol. Macromol. 16: 181–186.
Kaplan, N. O. (1955) Methods Enzmol. 2: 473.
Brady, T.G. and O’Sullivan, W. (1967) Biochim. Biophys. Acta 132: 127.
Dixon, M. and Webb, E.C. (1979) Enzymes 3rd Edn. pp. 61, Academic press, New York,.
Moosavi — Movahedi, A.A., Rahmani, Y. and Hakimelahi, G.H. (1993) Int. J. Biol. Macromol. 15: 125–129.
Moosavi-Movahedi, A.A., Samiee, B. and Hakimelahi, G.H. (1993) J. Colloid Interface Sci. 161: 53–59.
Hill, A.V. (1910) J. Physiol. 40: 4p.
Shimada, N., Hasegawa, S., Saito, S., Nishikiori, T., Fujii, A. and Takita, J. (1987) J Antibiotics 40: 1788.
Daddona, P.E., Schewach, D.S., William, N.K., Argos, P., Markham, A.F. and Orkin, S.H. (1984) J. Biol. Chem. 259: 1210.
Wilson, D.K., Rudolph, F.B. and Quiocho, F.A. (1991) Science 252: 1278–1281.
Moosavi-Movahedi, A.A., Moghaddamnia, H. and Hakimelahi, G.H. (1994) Iran. J. Chem. & Chem. Eng. 13:39–44.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1996 Plenum Press, New York
About this chapter
Cite this chapter
Moosavi-Movahedi, A.A. (1996). Thermodynamic Views of Inhibition, Activation and Denaturation of Adenosine Deaminase by Ring Opened Nucleosides and Denaturants. In: Zaidi, Z.H., Smith, D.L. (eds) Protein Structure — Function Relationship. Springer, Boston, MA. https://doi.org/10.1007/978-1-4613-0359-6_15
Download citation
DOI: https://doi.org/10.1007/978-1-4613-0359-6_15
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4613-8015-3
Online ISBN: 978-1-4613-0359-6
eBook Packages: Springer Book Archive