Abstract
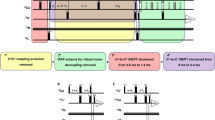
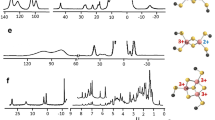
Newer NMR methods, particularly in conjunction with stable isotope labeling (with 2H, 13C, and 15N), offer powerful approaches to the elucidation of structure-function relationships in paramagnetic proteins such as iron-sulfur proteins. The optimization ofNMR pulse sequences for rapidly-relaxing spins and the utilization of multidimensional multinuclear NMR spectroscopy have made it possible to determine sequence-specific assignments for a large number of NMR signals in rubredoxins, ferredoxins, and high-potential iron proteins, including some from the cysteine residues that ligate the iron ions. Such assignments are the key to a wealth of information derived from NMR parameters, such as the temperature and pH dependence of chemical shifts and the relaxation properties of the resonances that report on interactions between nuclei of the protein and unpaired electron density from the metal center. This information can be used to test theoretical descriptions of electron distribution within these molecules and to model the structures and dynamic properties of the proteins in solution. Mutagenesis of these proteins, followed by biochemical evaluation of their functional properties and biophysical analysis of their structures and stabilities, is beginning to reveal which residues are important for cluster formation and which residues play a role in electron transfer to and from redox partner proteins. This review discusses recent NMR studies from our laboratory of six iron-sulfur proteins: Clostridium pasteurianum rubredoxin, the vegetative and heterocyst ferredoxins from Anabaena 7120, human ferredoxin, and the Pseudomonas mendocina KR1 tmoC Rieske protein.
Represents person presenting Paper
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Beinert, H. (1990) FASEB J. 4: 2483–2491.
Bruschi, M., and Guerlesquin, F. (1988) FEMS Microbiology Reviews 54: 155–176.
Howard, J. B. and Rees, D. C. (1991) Adv. Protein Chem. 42: 199–208.
Lovenberg, W., ed. (1973) Iron Sulfur Proteins, Vol. III, Academic Press, New York.
Spiro, T. G., ed. (1982) Iron Sulfur Proteins, Vol. IV, John Wiley, New York.
Sweeney, W. V. and Rabinowitz, J. C. (1980) Annu. Rev. Biochem. 49: 139–161.
Thompson, A. J. (1985) In: Metalloproteins (Hanson, P., ed.) Part I, pp. 79–120, Verlag Chemie, Weinheim.
Gurbiel, R. J., Batie, C. J., Sivaraja, M., True, A. E., Fee, J. A., Hoffman, B. M. and Ballou, D. P. (1989) Biochemistry 28: 4861–4871.
Cheng, H. and Markley J.L. (1995) Ann. Rev. Biophys. Biomol. Structure 24: 209–237.
Alam, J., Whitaker, R. A., Krogmann, D. W. and Curtis, S. E. (1986) J. Bacteriol. 168: 1265–1271
Böhme, H. and Haselkorn, R. (1988) Mol. Gen. Genet. 214: 278–285.
Mittal, S., Zhu, Y.-Z. and Vickery, L. E. (1988) Archives Biochem. Biophys. 264: 383–391.
Mathieu, I., Meyer, J. and Moulis, J.-M. (1992) Biochem. J. 285: 255–262.
Yen, K.-M., Karl, M. R., Blatt, L. M., Simon, M. J., Winter, R. B., Fausset, P. R., Lu, H. S., Harcourt, A. A. and Chen, K. K. (1991) J Bacteriol. 173: 5315–5327.
Cheng, H., Westler, W.M., **a, B., Oh, B.-H. and Markley, J.L. (1995) Arch. Biochem. Biophys. 316: 619–634.
Böhme, H. and Haselkorn, R. (1989) Plant Mol. Biol. 12: 667–672.
Chae, Y.K., Abildgaard, F., Mooberry, E.S. and Markley, J.L. (1994) Biochemistry 33: 3287–3295.
Coghlan, V. M. and Vickery, L. E. (1989) Proc. Natl. Acad. Sci. USA 86: 835–839.
**a, B., Cheng, H., Skjeldal, L., Coghlin, V.M., Vickery, L.E. and Markley, J.L. (1995) Biochemistry 34: 180–187.
**a, B., Westler, W. M., Cheng, H, Meyer, J., Moulis, J.-M. and Markley, J. L. (1995) J. Am. Chem. Soc., in press.
Fox, B. G. and Pikus, J. D. unpublished results.
Phillips, W. D. (1973) In: NMR of Paramagnetic Molecules, eds. (La Mar, G.N., Horrocks, W.D., Holm R.H., eds.) pp. 421–478. Academic Press, New York.
Phillips, W. D. and Poe, M. (1972) In: Methods in Enzymology,(San Pietro, A. ed.) vol. 24: pp. 304–317, Academic Press, New York.
Phillips, W. D. and Poe, M. (1973) In: Iron-Sulfur Proteins,(Lovenberg, W. ed) vol. 2: pp. 255–284, Academic Press, New York.
Bertini, I., and Luchinat, C. (1986) NMR of Paramagnetic Molecules in Biological Systems, Benjamin/Cummings, Menlo Park, California.
Bertini, I., Banci, L. and Luchinat, C. (1989) Methods Enzymol. 177: 246–264.
Blake, P.R., Park, J.-B., Adams, M. W. W. and Summers, M. F. (1992) J. Am. Chem. Soc. 114: 4931–4933.
Markley, J. L., Chan, T.-M, Krishnamoorthi, R., & Ulrich, E. L. (1986) In: Iron-Sulfur Protein Research, (Matsubara, H., Katsube, Y., Wada, K. eds.) pp. 167–181, Springer-Verlag, Tokyo/Berlin.
Nagayama, K, Ohmori, D., Imai, T., & Oshima, T. (1986) In: Iron-Sulfur Protein Research,(Matsubara, H., Katsube, Y., Wada, K. eds.) pp. 125–138, Springer-Verlag, Tokyo/Berlin.
Krishnamoorthi, R., Markley, J. L., Cusanovich, M. A., Przysiecki, C. T. and Meyer, T. E. (1986) Biochemistry 25: 60–67.
Dugad, L. B., Le Mar, G. N., Banci, L. and Bertini, I. (1990) Biochemistry 29: 2263–2271.
Bertini, I. (1994) The Tridimensional Structure of a Paramagnetic HiPIP in Solution from NMR, International Conference on the Use of Stable Isotopes in NMR Studies of Protein Structure, Dynamics and Function, Palais du Luxembourg, Paris, March 21–23, abstract.
Bertini, I., Briganti, F., Luchinat, C. and Scozzafava, A. (1990) I H NMR Studies of the Oxidized and Partially Reduced 2[Fe-4S] Ferredoxins from Clostridium pasteurianum. Inorg. Chem. 28: 1874–1880.
Bertini, I., Briganti, F., Luchinat, C., Messori, L., Monnanni, R., Scozzafava, A. and Vallini, G. (1991) FEBS Lett. 289: 253–256.
Bertini, I., Briganti, F., Luchinat, C., Messori, L., Monnanni, R., Scozzafava, A. and Vallini, G. (1992) Eur J. Biochem. 28: 1874–1880.
Busse, S. C., La Mar, G. N. and Howard, J. B. (1991) J. Biol. Chem. 266: 23714–23723.
Gaillard, J., Moulis, J.-M. and Meyer, J. (1987) Inorg. Chem. 26: 320–324.
Pochapsky, T. C. and Ye, X. M. (1991) Biochemistry 30: 3850–3856.
Ye, X. M., Pochapsky, T. C. and Pochapsky, S. S. (1992) Biochemistry 31: 1961–1968.
Greenfield, J. J., Wu, X. and Jordan (1989) Biochim. Biophys. Acta 995: 246–254.
Miura, S. and Ichikawa, Y. (1991) Eur. J. Biochem. 197: 747–757.
Krishnamoorthi, R., Markley, J. L., Cusanovich, M. A. and Przysiecki, C. T. (1986) Biochemistry 25: 50–54.
Banci, L., Bertini, I. and Briganti, F. (1991) New J. Chem. 15: 467–477.
Satterlee, J. D. (1990) Concepts in Magn. Reson. 2: 69–79 and 119–129.
Bertini, I., Turano, P. and Vila, A. (1993) Chem. Rev. 93: 2833–2932.
Wüthrich, K. (1986) NMR of Proteins and Nucleic Acids, Wiley, New York.
Edison, A. S., Abildgaard, F., Westler, W. M., Mooberry, E. S. and Markley, J. L. (1994) Methods. Enzymol. 239: 3–79.
Lecomte, J. T. J., Unger, S. W. and La Mar, G. N. (1991) J. Magn. Reson. 94: 112–122.
Wimperis, S. and Bodenhausen, G. (1989) Mol. Physics 66: 897–919.
Qin, J., Delaglio, F., La Mar, G. and Bax, A. (1993) J. Magn. Reson. Ser. B 102: 332–336.
Bertini, I., Luchinat, C. and Tarchi, D. (1993) Chem. Phys. Lett. 203: 445–449.
Werth, M. T., Kurtz, D. M., Jr., Moura, I. and LeGall, J. (1987) J Am. Chem. Soc. 109: 273–275.
Cheng, H., **a, B., Reed, G. H. and Markley, J. L. (1994) Biochemistry 33: 3155–3164.
Holden, H. M., Jacobson, B. L., Hurley, J. K., Tollin, G., Oh, B.-H., Skjeldal, L., Chae, Y. K., Cheng, H., **a, B. and Markley, J. L. (1994) J. Bioeng. Biomemb. 26: 67–88.
Hurley, J. K., Salamon, Z., Meyer, T. E., Fitch, J. C., Cusanovich, M. A., Markley, J. L., Cheng, H., **a, B., Chae, Y. K., Medina, M., Gomez-Moreno, C. and Tollin, G. (1993) Biochemistry 32: 9346–9354.
Hurley, J. K., Cheng, H., **a, B., Markley, J. L., Medina, M., Gomez-Moreno, C. and Tollin, G. (1993) J. Am. Chem. Soc. 115 11698–11701.
Rypniewski, W. R. Breiter, D. R., Benning, M. M., Wesenberg, G., Oh, B.-H., Markley, J. L., Rayment, I. and Holden, H. M. (1991) Biochemistry 30: 4126–4131.
Miura, S., Tomita, S. and Ichikawa, Y. (1991) J. Biol. Chem. 266: 19212–19216.
Miura, S. and Ichikawa, Y. (1991) J. Biol. Chem. 266: 6252–6258.
Chae, Y. K., **a, B., Cheng, H., Oh, B.-H., Skjeldal, L., Westler, W. M. and Markley, J. L. (1995) In: Nuclear Magnetic Resonance of Paramagnetic Macromolecules (La Mar, G.N. ed.), NATO ASI Series C, Vol. 457: pp. 297–317, Kluwer, Dordrecht.
Chae, Y. K. (1994) Multinuclear Multidimensional NMR Studies of Anabaena 7120 vegetative and Heterocyst Ferredoxins, Ph.D. Thesis, University of Wisconsin-Madison.
Breiter, D. R., Meyer, T. E., Rayment, I. and Holden, H. M. (1991) J. Biol. Chem. 266: 18660–18667.
Chae, Y. K. and Markley, J. L. (1995) Biochemistry 34: 188–193.
Hurley, J. K., Caffrey, M. S., Markley, J. L., Cheng, H., **a, B., Chae, Y. K., Holden, H. M. and Tollin, G. Protein Sci., in press.
Fee, J. A., Findling, K. L., Yôshida, T., Hille, R., Tan, G. E., Hearshen, D. O., Dunham, W. R., Day, E. P., Kent, T. A. and Münck, E. (1984) J. Biol. Chem., 259: 124–133.
Altier, D. J., Fox, B. G., Münck, E. and Lipscomb, J. D. (1993) J. Inorg. Biochem. 43: 300.
Gurbiel, R. J., Ohnishi, T., Robertson, D. E., Daldal, F. and Hoffman, B. M. (1991) Biochemistry 30: 11579–11584.
Dunham, W. R., Palmer, G., Sands, R. H. and Bearden, A. J. (1971) Biochim. Biophys. Acta 253: 373–384.
Banci, L., Bertini, I. and Luchinat, C. (1990) Struct. Bonding 72: 113–136.
Skjeldal, L., Westler, W. M. and Markley, J. L. (1990) Arch. Biochem. Biophys. 278: 482–485.
Skjeldal, L., Markley, J. L., Coghlan, V. M. and Vickery, L. (1991) Biochemistry 30: 9078–9083.
Skjeldal, L., Westler, W. M., Oh, B.-H., Krezel, A. M., Holden, H. M., Jacobson, B. L., Rayment, I. and Markley, J. L. (1991) Biochemistry 30: 7363–7368.
Banci, L., Bertini, I., Briganti, F., Luchinat, C. and Scozzafava, A., et al. (1991) Inorg. Chem. 30: 4517–4524.
Banci, L., Bertini, I., Briganti, F., Scozzafava, A. and Oliver, M. V. (1991) Inorg. Chim. Acta 180: 171–175.
Banci, L., Bertini, I., Capozzi, F., Carloni, P. and Ciurli, S. et al. (1992) J. Am. Chem. Soc. 115: 3431–3440.
Banci, L., Bertini, I., Ciurli, S., Ferretti, S. and Luchinat, C. et al. (1993) Biochemistry 32: 9387–9397.
Bertini, I., Capozzi, F., Ciurli, S., Luchinat, C., Messori, L. and Piccioli, M. (1992) J. Am. Chem. Soc. 114: 3332–3340.
Bertini, I., Capozzi, F., Luchinat, C., Piccioli, M. and Oliver, V. (1992) Inorg. Chim. Acta 198–200, 483–491.
Bertini, I., Capozzi, F., Luchinat, C. and Piccioli, M. (1993) Eur. J. Biochem. 212: 69–78.
Busse, S. C., La Mar, G. N., Yu, L. P., Howard, J. B. and Smith, E. T., et al. (1992) Biochemistry 31: 11952–11962.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1996 Plenum Press, New York
About this chapter
Cite this chapter
Markley, J.L. et al. (1996). NMR Approaches to the Study of Structure-Function Relationships in Iron-Sulfur Proteins. In: Zaidi, Z.H., Smith, D.L. (eds) Protein Structure — Function Relationship. Springer, Boston, MA. https://doi.org/10.1007/978-1-4613-0359-6_14
Download citation
DOI: https://doi.org/10.1007/978-1-4613-0359-6_14
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4613-8015-3
Online ISBN: 978-1-4613-0359-6
eBook Packages: Springer Book Archive