Abstract
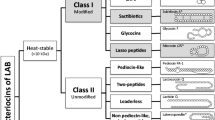
Lactic acid bacteria (LAB) are known to produce antibacterial peptides and small proteins called bacteriocins, which enable them to compete against other bacteria in the environment. Bacteriocins fall structurally and chemically into three different classes, I, II, and III. Bacteriocins are ribosomally synthesized peptides with antagonism against closely related bacteria. This late observation has evolved because bacteriocins active against Gram-negative bacteria have recently been reported. Members of class IIa bacteriocins, referred to as pediocin-like bacteriocins, are among the most studied bacteriocins. This chapter is aimed at providing an updated review on the biology of class IIa bacteriocins.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Atrih A, Rekhif N, Moir AJ, Lebrihi A, Lefebvre G (2001) Mode of action, purification and amino acid sequence of plantaricin C19, an anti-Listeria bacteriocin produced by Lactobacillus plantarum C19. Int J Food Microbiol 68:93–104
Aymerich T, Holo H, Håvarstein LS, Hugas M, Gariga M, Nes IF (1996) Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl Environ Microbiol 62:1676–1682
Belguesmia Y, Madi A, Sperandio D, Merieau A, Feuilloley M, Prévost H, Drider D, Connil N (2010) Growing insights into safety of bacteriocins: case of enterocin S37. Res Microbiol In press
Bennik MHJ, Vanloo B, Brasseur R, Gorris LGM, Smid EJ (1998) A novel bacteriocin with a YGNGV motif from vegetable-associated Enterococcus mundtii: full characterization and interaction with target organisms. Biochim Biophys Acta 1373:47–58
Bhunia AK, Johnson MC, Ray B, Kalchayanand N (1991) Mode of action of pediocin AcH from Pediococcus acidilactici H on sensitive bacterial strains. J Appl Bacteriol 70:25–33
Birri DJ, Brede DA, Forberg T, Holo H, Nes IF (2010) Molecular and genetic characterization of a novel bacteriocin locus in Enterococcus avium isolates from infants. Appl Environ Microbiol 76:483–492
Calvez S, Rincé A, Auffray Y, Prévost H, Drider D (2007) Identification of new genes associated with intermediate resistance of Enterococcus faecalis to divercin V41, a pediocin-like bacteriocin. Microbiology 153:1609–1618
Calvez S, Prévost H, Drider D (2008a) Relative expression of genes involved in the resistance/sensitivity of Enterococcus faecalis JH2-2 to recombinant divercin RV41. Biotechnol Lett 30:1795–1800
Calvez S, Prévost H, Drider D (2008b) Identification of a new molecular target of class IIa bacteriocins in Listeria monocytogenes EGDe. Folia Microbiol (Praha) 53:417–422
Calvez S, Kohler A, Prévost H, Møretrø T, Drider D (2010) Physiological and structural differences between Enterococcus faecalis JH2-2 and mutant strains resistant to (P)-divercin RV41. Probiotics Antimicro Prot. doi:DOI 10.1007/s12602-010-9048-1 (Published online)
Chang C, Cogill P, Bateman A, Finn RD, Cymorowski M, Otwinowski Z, Minor W, Volkart L, Joachim A (2009) The structure of pyogenecin immunity proytein, a novel bacteriocn-like immunity protein from Streptococcus pyogenes. BMC Struct Biol 17:9–75
Chen Y, Ludescher RD, Montville TJ (1997a) Electrostatic interactions, but not the YGNGV consensus motif, govern the binding of pediocin PA-1 and its fragments to phospholipid vesicles. Appl Environ Microbiol 63:4770–4777
Chen Y, Shapira R, Eisenstein M, Montville TJ (1997b) Functional characterization of pediocin PA-1 binding to liposomes in the absence of a protein receptor and its relationship to a predicted tertiary structure. Appl Environ Microbiol 63:524–531
Chikindas ML, Garcia-Garcera MJ, Driessen AJ, Ledeboer AM, Nissen-Meyer J, Nes IF, Abee T, Konings WN, Venema G (1993) Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl Environ Microbiol 59:3577–3584
Cho HS, Pelton JG, Yan D, Kustu S, Wemmer DE (2001) Phosphoaspartates in bacterial signal transduction. Curr Opin Struct Biol 11:679–684
Christensen DP, Hutkins RW (1992) Collapse of the proton motive force in Listeria monocytogenes caused by a bacteriocin produced by Pediococcus acidilactici. Appl Environ Microbiol 58:3312–3315
Cintas LM, Casaus P, Håvarstein LS, Hernández PE, Nes IF (1997) Biochemical and genetical characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl Environ Microbiol 63:4321–4330
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol 71:1–20
Dabour N, Zihler A, Kheadr E, Lacroix C, Fliss I (2009) In vivo study on the effectiveness of pediocin PA-1 and Pediococcus acidilactici UL5 at inhibiting Listeria monocytogenes. Int J Food Microbiol 133:225–233
Dalet K, Briand C, Cenatiempo Y, Héchard Y (2000) The rpoN gene of Enterococcus faecalis directs sensitivity to subclass IIa bacteriocins. Curr Microbiol 41:441–443
Dalet K, Cenatiempo Y, Cossart P, Hechard Y (2001) European Listeria Genome Consortium. A sigma (54)-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiology 147:3263–3269
De Kwaadsteniet M, Fraser T, Van Reenen CA, Dicks LMT (2006) Bacteriocin T8, a novel class Iia sec-dependent bacteriocin produced by Enterococcus faecium T8, isolated from vaginal secretions of children infected with human immunodeficiency virus. Appl Environ Microbiol 72:4761–4766
De la Cruz I, Davies I (2000) Horizontal gene transfer and the origin of species: lessons from bacteria. Trends Microbiol 8:128–133
Del Campo R, Tenorio C, Jimenez-Diaz R, Rubio C, Gomez-Lus R, Baquero F, Torres C (2001) Bacteriocin production in vancomycin-resistant and vancomycin-susceptible Enterococcus isolates of different origins. Antimicrob Agents Chemother 45:905–912
Diep DB, Godager L, Brede D, Nes IF (2006) Data mining and characterization of a novel pediocin-like bacteriocin system from the genome of Pediococcus pentosaceus ATCC 25745. Microbiology 152:1649–1659
Diep DB, Skaugen M, Salehian Z, Holo H, Nes IF (2007) Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc Natl Acad Sci USA 104:2384–2389
Drider D, Fimland G, Héchard Y, McMullen LM, Prévost H (2006) The continuing story of class IIa bacteriocins. Microbiol Mol Biol Rev 70:564–582
Eguchi T, Kaminaka K, Shima J, Kawamoto S, Mori K, Choi SH, Doi K, Ohmomo S, Ogata S (2001) Isolation and characterization of enterocin SE-K4 produced by thermophilic enterococci, Enterococcus faecalis K-4. Biosci Biotechnol Biochem 65:247–253
Ennahar S, Sashihara T, Sonomoto K, Ishizaki A (2000) Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol Rev 24:85–106
Feng G, Guron GK, Churey JJ, Worobo RW (2009) Characterization of mundticin L, a class IIa anti-Listeria bacteriocin from Enterococcus mundtii CUGF08. Appl Environ Microbiol 75:5708–5713
Ferchichi M, Frère J, Mabrouk K, Manai M (2001) Lactococcin MMFII, a novel class IIa bacteriocin produced by Lactococcus lactis MMFII, isolated from a Tunisian dairy product. Lett Appl Microbiol 205:49–55
Fimland G, Jack R, Jung G, Nes IF, Nissen-Meyer J (1998) The bactericidal activity of pediocin PA-1 is specifically inhibited by a 15-mer fragment that spans the bacteriocin from the center toward the C terminus. Appl Environ Microbiol 64:5057–5060
Fimland G, Eijsink VG, Nissen-Meyer J (2002) Comparative studies of immunity proteins of pediocin-like bacteriocins. Microbiology 148:3661–3670
Fimland G, Johnsen L, Dalhus B, Nissen-Meyer J (2005) Pediocin-like antimicrobial peptides (class IIa bacteriocins) and their immunity proteins: biosynthesis, structure and mode of action. J Pept Sci 11:688–696
Fimland G, Pirneskoski J, Kaewsrichan J, Jutila A, Kristiansen PE, Kinnunen PKJ, Nissen-Meyer J (2006) Mutational analysis and membrane-interactions of the beta-sheet-like N-terminal domain of the pediocin-like antimicrobial peptide sakacin P. Biochim Biophys Acta 1764:1132–1140
Fleury Y, Dayem MA, Montagne JJ, Chaboisseau E, Le Caer JP, Nicolas P, Delfour A (1996) Covalent structure, synthesis, and structure-function studies of mesentericin Y 105(37), a defensive peptide from gram-positive bacteria Leuconostoc mesenteroides. J Biol Chem 271:114421–114429
Frégeau Gallagher NL, Sailer M, Niemczura WP, Nakashima TT, Stiles ME, Vederas JC (1997) Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type IIa bacteriocins from lactic acid bacteria. Biochemistry 36:15062–15072
Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, Baquero F, Berche P, Bloecker H, Brandt P, Chakraborty T, Charbit A, Chetouani F, Couvé E, de Daruvar A, Dehoux P, Domann F, Domínguez-Bernal G, Duchaud E, Durant L, Dussurget O, Entian KD, Fsihi H, García-del Portillo F, Garrido P, Gautier L, Goebel W, Gómez-López N, Hain T, Hauf J, Jackson D, Jones LM, Kaerst U, Kreft J, Kuhn M, Kunst F, Kurapkat G, Madueno F, Maitournam A, Vicente JM, Ng E, Nedjari H, Nordsiek G, Novella S, de Pablos B, Pérez-Diaz JC, Purcell R, Remmel B, Rose M, Schlueter T, Simoes N, Tierrez A, Vázquez-Boland JA, Voss H, Wehland J, Cossart P (2001) Comparative genomics of Listeria species. Science 294:849–852
Gravesen A, Ramnath M, Rechinger KB, Andersen N, Jansch L, Héchard Y, Hastings JW, Knochel S (2002) High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiology 148:2361–2369
Hauge HH, Mantzilas D, Moll GN, Konings WN, Driessen AJM, Eijsink VGH, Nissen-Meyer J (1998) Plantaricin A is an amphiphilic α-helical bacteriocin-like pheromone which exerts antimicrobial and pheromone activities through different mechanisms. Biochemistry 37:16026–16032
Haugen HS, Fimland G, Nissen-Meyer J, Kristiansen PE (2005) Three-dimensional structure in lipid micelles of the pediocin-like antimicrobial peptide curvacin A. Biochemistry 44:16149–16157
Haugen HS, Kristiansen PE, Fimland G, Nissen-Meyer J (2008) Mutational analysis of the class IIa bacteriocin curvacin A and its orientation in target cell membranes. Appl Environ Microbiol 74:6766–6773
Héchard Y, Pelletier C, Cenatiempo Y, Frère J (2001) Analysis of sigma(54)-dependent genes in Enterococcus faecalis: a mannose PTS permease (EII(Man)) is involved in sensitivity to a bacteriocin, mesentericin Y105. Microbiology 147:1575–1580
Henderson JT, Chopko AL, van Wassenaar PD (1992) Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC-1.0. Arch Biochem Biophys 295:5–12
Heng NCK, Burtenshaw GA, Jack RW, Tagg JR (2007) Ubericin A, a Class IIa bacteriocin produced by Streptococcus uberis. Appl Environ Microbiol 73:7763–7766
Herranz C, Chen Y, Chung HJ, Cintas LM, Hernández PE, Montville TJ, Chikindas ML (2001a) Enterocin P selectively dissipates the membrane potential of Enterococcus faecium T136. Appl Environ Microbiol 67:1689–1692
Herranz C, Cintas LM, Hernández PE, Moll GN, Driessen AJ (2001b) Enterocin P causes potassium ion efflux from Enterococcus faecium T136 cells. Antimicrob Agents Chemother 45:901–904
Hühne K, Axelsson L, Holck A, Kröckel L (1996) Analysis of the sakacin P gene cluster from Lactobacillus sake Lb674 and its expression in sakacin-negative Lb. sake strains. Microbiology 142:1437–1448
Ingham A, Ford M, Moore RJ, Tizard M (2003) The bacteriocin piscicolin 126 retains antilisterial activity in vivo. J Antimicrob Chemother 51:1365–1371
Jack RW, Wan J, Gordon J, Harmark K, Davidson BE, Hillier AJ (1996) Characterization of the chemical and antimicrobial properties of piscicolin 126, a bacteriocin produced by Carnobacterium piscicola JG126. Appl Environ Microbiol 62:2897–2903
Joerger RD (2003) Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poult Sci 82:640–647
Johnsen L, Dalhus B, Leiros I, Nissen-Meyer J (2005) 1.56 Å crystal structure of entA-im: a bacterial immunity protein conferring immunity to the antimicrobial activity of the pediocin-like bacteriocin enterocin A. J Biol Chem 280:19045–19050
Kaiser AL, Montville TJ (1996) Purification of the bacteriocin bavaricin MN and characterization of its mode of action against Listeria monocytogenes Scott A cells and lipid vesicles. Appl Environ Microbiol 62:4529–4535
Kalmokoff ML, Banerjee SK, Cyr TD, Hefford MA, Gleeson T (2001) Identification of a new plasmid-encoded sec-dependent bacteriocin produced by Listeria innocua 743. Appl Environ Microbiol 67:4041–4047
Kaur K, Andrew LC, Wishart DS, Vederas JC (2004) Dynamic relationships among type IIa bacteriocins: temperature effects on antimicrobial activity and on structure of the C-terminal amphipathic_helix as a receptor binding region. Biochemistry 43:9009–9020
Kawamoto S, Shima J, Sato R, Eguchi T, Ohmomo S, Shibato J, Horikoshi N, Takeshita K, Sameshima T (2002) Biochemical and genetic characterization of mundticin KS, an antilisterial peptide produced by Enterococcus mundtii NFRI 7393. Appl Environ Microbiol 68:3830–3840
Kazazic M, Nissen-Meyer J, Fimland G (2002) Mutational analysis of the role of charged residues in target-cell binding, potency and specificity of the pediocin-like bacteriocin sakacin P. Microbiology 148:2019–2027
Kim IK, Kim MK, Kim JH, Yim HS, Cha SS, Kang SO (2007) High resolution crystal structure of PedB: a structural basis for the classification of pediocin-like immunity proteins. BMC Struct Biol 30(7):35
Kjos M, Nes IF, Diep DB (2009) Class II one-peptide bacteriocins target a phylogenetically defined subgroup of mannose phosphotransferase systems on sensitive cells. Microbiology 155:2949–2961
Kjos M, Salehian Z, Nes IF, Diep DB (2010) An extracellular loop of the mannose phosphotransferase 1 system component IIC is responsible for specific targeting by class IIa bacteriocins. J Bacteriol
Kristiansen PE, Fimland G, Mantzilas D, Nissen-Meyer J (2005) Structure and mode of action of the membrane permeabilizing antimicrobial peptide pheromone plantaricin A. J Biol Chem 280:22945–22950
Lasta S, Fajloum Z, Darbon H, Mansuelle P, Andreotti N, Sabatier JM, Boudabous A, Sampieri F (2008) Chemical synthesis and characterization of J46 peptide, an atypical class IIa bacteriocin from Lactococcus lactis subsp. cremoris J46 strain. J Antibiot (Tokyo) 61:89–93
Le Marrec C, Hyronimus B, Bressollier P, Verneuil B, Urdaci MC (2000) Biochemical and genetic characterization of coagulin, a new antilisterial bacteriocin in the pediocin family of bacteriocins, produced by Bacillus coagulans. Appl Environ Microbiol 66:5213–5220
Li JW, Vederas JC (2009) Drug discovery and natural products: end of an era or an endless frontier? Science 325:161–165
Line JE, Svetoch EA, Eruslanov BV, Perelygin VV, Motsevich EV, Mitsevich IP, Levchuk VP, Svetoch OE, Seal BE, Siragusa GR, Stern NJ (2008) Isolation and purification of enterocin E-760 with broad antimicrobial activity against gram-positive and gram-negative bacteria. Antimicrob Agents Chemother 52:1094–1100
Maftah A, Renault D, Vignoles C, Héchard Y, Bressollier P, Ratinaud MH, Cenatiempo Y, Julien R (1993) Membrane permeabilization of Listeria monocytogenes and mitochondria by the bacteriocin mesentericin Y105. J Bacteriol 175:3232–3235
Martin-Visscher LA, Sprules T, Gursky LJ, Vederas J (2008) Nuclear magnetic resonance solution structure of PisL, a group B immunity protein that provides protection against the type IIa bacteriocin piscicolin 126, PisA. Biochemistry 47:6427–6436
Métivier A, Pilet MF, Dousset X, Sorokine O, Anglade P, Zagorec M, Picard JC, Marion D, Cenatiemp Y, Frémaux C (1998) Divercin V41, a new bacteriocin with two disulphide bonds produced by Carnobacterium divergens V41: primary structure and genomic organization. Microbiology 144:2837–2844
Miller KW, Schamber R, Osmanagaoglu O, Ray B (1998a) Isolation and characterization of pediocin AcH chimeric protein mutants with altered bactericidal activity. Appl Environ Microbiol 64:1997–2005
Miller KW, Schamber R, Chen Y, Ray B (1998b) Production of active chimeric pediocin AcH in Escherichia coli in the absence of processing and secretion genes from the Pediococcus pap operon. Appl Environ Microbiol 64:14–20
Miller KW, Ray P, Steinmetz T, Hanekamp T, Ray B (2005) Gene organization and sequences of pediocin AcH/PA-1, production operons in Pediococcus and Lactococcus plasmids. Lett Appl Microbiol 40:52–62
Minahk CJ, Dupuy F, Morero RD (2004) Enhancement of antibiotic activity by sub-lethal concentrations of enterocin CRL35. J Antimicrob Chemother 54:240–246
Morisset D, Berjeaud JM, Marion D, Lacombe C, Frère J (2004) Mutational analysis of mesentericin Y105 an anti-Listeria bacteriocin, for determination of impact on bactericidal activity, in vitro secondary structure, and membrane interaction. Appl Environ Microbiol 70:4672–4680
Naghmouchi K, Drider D, Fliss I (2007) Action of divergicin M35, a class IIa bacteriocin, on liposomes and Listeria. J Appl Microbiol 102:1508–1517
Naghmouchi K, Belguesmia Y, Baah J, Ron T, Drider D (2010a) Antibacterial activity of class I and IIa bacteriocins combined with polymyxin E against resistant variants of Listeria monocytogenes and Escherichia coli. Res Microbiol In press
Naghmouchi K, Drider D, Baah J, Teather R (2010b) Nisin A and polymyxin B as synergistic inhibitors of Gram-positive and Gram-negative bacteria. Probiotics Antimicrob Proteins 1–2:98–103
Nes IF, Eijsink VGH (1999) Regulation of group II peptide bacteriocin synthesis by quorum-sensing mechanisms. In: Dunny GM, Winans SC (eds) Cell–cell signalling in bacteria. American Society for Microbiology, Washington, DC, pp 175–192
Nes IF, Diep DB, Havarstein LS, Brurberg MB, Eijsink V, Holo H (1996) Biosynthesis of bacteriocins in lactic acid bacteria. Antoine Van Leeuwenhoek 70:113–128
Nieto-Lozano JC, Reguera-Useros JI, Peláez-Martínez MC, Hardisson de la Torre A (2006) Effect of a bacteriocin produced by Pediococcus acidilactici against Listeria monocytogenes and Clostridium perfringens on Spanish raw meat. Meat Sci 72:57–61
Nissen-Meyer J, Rogne P, Oppegard C, Haugen HS, Kristiansen PE (2009) Structure-function relationships of the non-lanthionine-containing peptide (class II) bacteriocins produced by gram-positive bacteria. Curr Pharm Biotechnol 10:19–37
O’Keeffe T, Hill C, Ross R (1999) Characterization and heterologous expression of the genes encoding enterocin A production, immunity, and regulation in Enterococcus faecium DPC1146. Appl Environ Microbiol 65:1506–1515
O’Mahony AT, O’Sullivan FO, Walsh Y, Vaughan A, Maher M, Fitzerald GF, Van Sindersen D (2000) Characterization and heterologous of antimicrobial producing lactic acid bacteria from malted barley. J Inst Brew 106:403–410
Papathanasopoulos MA, Dykes GA, Revol-Junelles AM, Delfour A, von Holy A, Hastings JW (1998) Sequence and structural relationships of leucocins A-, B-, and C-TA33a from Leuconostoc mesenteroides TA33a. Microbiology 144:1343–1348
Postma PW, Langeler JW, Jacobson GR (1993) Phosphoenolpyruvate: carbohydrate phosphotransferase systems of bacteria. Microbiol Rev 57:543–594
Quadri LEN, Sailer M, Roy KL, Vederas JC, Stiles ME (1994) Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J Biol Chem 269:12204–12211
Quadri LEN, Kleerebezem M, Kuipers OP, de Vos WM, Roy KL, Vederas JC, Stiles ME (1997) Characterization of a locus from Carnobacterium piscicola LV17B involved in bacteriocin production and immunity: evidence for global inducer-mediated transcriptional regulation. J Bacteriol 179:6163–6171
Ramnath M, Arous S, Gravesen A, Hastings JW, Héchard Y (2004) Expression of mptC of Listeria monocytogenes induces sensitivity to class IIa bacteriocins in Lactococcus lactis. Microbiology 150:2663–2668
Rea MC, Sit CS, Clayton E, O’Connor PM, Whittal RM, Zheng J, Vederas JC, Ross RP, Hill C (2010) Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc Natl Acad Sci U S A 107:9352–9357
Richard C, Brillet A, Pilet MF, Prévost H, Drider D (2003) Evidence on inhibition of Listeria monocytogenes by divercin V41 action. Lett Appl Microbiol 36:288–292
Richard C, Drider D, Elmorjani K, Marion D, Prévost H (2004) Heterologous expression and purification of active divercin V41, a class IIa bacteriocin encoded by a synthetic gene in Escherichia coli. J Bacteriol 186:4276–4284
Richard C, Cañon R, Naghmouchi K, Bertrand D, Prévost H, Drider D (2006) Evidence on correlation between number of disulfide bridge and toxicity of class IIa bacteriocins. Food Microbiol 23:175–183
Rihakova J, Petit VW, Demnerova K, Prévost H, Rebuffat S, Drider D (2009a) Insights into structure-activity relationships in the C-terminal region of divercin V41, a class IIa bacteriocin with high-level antilisterial activity. Appl Environ Microbiol 75:1811–1819
Rihakova J, Belguesmia Y, Petit VW, Pilet MF, Prévost H, Dousset X, Drider D (2009b) Divercin V41 from gene characterization to food applications: 1998–2008, a decade of solved and unsolved questions. Lett Appl Microbiol 48:1–7
Rihakova J, Cappelier JM, Hue I, Demnerova K, Fédérighi M, Prévost H, Drider D (2010). Antimicrob Agents Chemother. 54: 563–564
Robichon D, Gouin E, De’barbouille M, Cossart P, Cenatiempo Y, Héchard Y (1997) The rpoN (σ54) gene from Listeria monocytogenes is involved in resistance to mesentericin Y105, an antibacterial peptide from Leuconostoc mesenteroides. J Bacteriol 179:7591–7594
Rodríguez JM, Martínez MI, Kok J (2002) Pediocin PA-1, a wide-spectrum bacteriocin from lactic acid bacteria. Crit Rev Food Sci Nutr 42:91–121
Salvucci E, Saavedra L, Sesma F (2007) Short peptides derived from the NH2-terminus of subclass IIa bacteriocin enterocin CRL35 show antimicrobial activity. J Antimicrob Chemother 59:1102–1108
Simon L, Frémaux C, Cenatiempo Y, Berjeaud JM (2002) Sakacin G, a new type of antilisterial bacteriocin. Appl Environ Microbiol 12:6416–6420
Skaugen M, Cintas LM, Nes IF (2003) Genetics of bacteriocin production in lactic acid bacteria. In: Wood BJB, Warner PJ (eds) Genetics of lactic acid bacteria. Kluwer Academic/Plenum Publishers, New York, NY, pp 225–260
Smoaki W (1999) Cromakalim: Embryonic effects and reduction of tolbutamide-induced dysmorphogenesis in vitro. Teratology 60:260–264
Soliman W, Bhattacharjee S, Kaur K (2007) Molecular dynamics simulation study of interaction between a class IIa bacteriocin and its immunity protein. Biochim Biophys Acta 1774:1002–10013
Sprules T, Kawulka KE, Gibbs AC, Wishart DS, Vederas JC (2004) NMR solution structure of the precursor for carnobacteriocin B2, an antimicrobial peptide from Carnobacterium piscicola. Eur J Biochem 271:1748–1756
Stern NJ, Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, Pokhilenko VD, Levchuk VP, Svetoch OE, Seal BS (2006) Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to campylobacter jejuni in the chicken gastrointestinal system. Antimicrob Agents Chemother 50:3111–3116
Straume D, Kjos M, Nes IF, Diep DB (2007) Quorum-sensing based bacteriocin production is down-regulated by N-terminally truncated species of gene activators. Mol Genet Genomics 278:283–293
Svetoch EA, Stern NJ (2010) Bacteriocins to control Campylobacter spp. in poultry. Poult Sci 89:1763–1768
Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, Borzenkov VN, Levchuk VP, Svetoch OE, Kovalev YN, Stepanshin YG, Siragusa GR, Seal BS, Stern NJ (2008a) Diverse antimicrobial killing by Enterococcus faecium E 50-52 bacteriocin. J Agric Food Chem 56:1942–1948
Svetoch EA, Levchuk VP, Pokhilenko VD, Eruslanov BV, Mitsevich EV, Mitsevich IP, Perelygin VV, Stepanshin YG, Stern NJ (2008b) Inactivating methicillin-resistant Staphylococcus aureus and other pathogens by use of bacteriocins OR-7 and E 50-52. J Clin Microbiol 46:3863–3865
Tahiri I, Desbiens M, Benech R, Kheadr E, Lacroix C, Thibault S, Ouellet D, Fliss I (2004) Purification, characterization and amino acid sequencing of divergicin M35: a novel class IIa bacteriocin produced by Carnobacterium divergens M35. Int J Food Microbiol 97:123–136
Taylor WR (1986) The classification of amino acid conservation. J Theor Biol 119:205–218
Tessema GT, Moretro T, Kohler A, Axelsson L, Naterstad K (2009) Complex phenotypic and genotypic responses of Listeria monocytogenes strains exposed to the class IIa bacteriocin sakacin P. Appl Environ Microbiol 75:6973–6980
Tichaczek PS, Nissen-Meyer J, Nes IF, Vogel RF, Hammes WP (1992) Characterization of the bacteriocins curvacin A from Lactobacillus curvatus LTH 1174 and Sakacin P from L. sake LTH 673. Syst Appl Microbiol 15:460–468
Tichaczek PS, Vogel RF, Hammes WP (1994) Cloning and sequencing of sakP encoding sakacin P, the bacteriocin produced by Lactobacillus sake LTH 673. Microbiology 140:361–367
Todorov SD, Wachsman M, Tomé E, Dousset X, Destro MT, Dicks LM, Franco BD, Vaz-Velho M, Drider D (2010) Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiol 27:869–879
Tominaga T, Hatakeyama Y (2007) Development of innovative pediocin PA-1 by DNA shuffling among class IIa bacteriocins. Appl Environ Microbiol 73:5292–5299
Tomita H, Fujimoto S, Tanimoto K, Ike Y (1996) Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI17. J Bacteriol 178:3585–3593
Uteng M, Hauge HH, Markwick PR, Fimland G, Mantzilas D, Nissen-Meyer J, Muhle-Goll C (2003) Three-dimensional structure in lipid micelles of the pediocin-like antimicrobial peptide sakacin P and a sakacin P variant that is structurally stabilized by an inserted C-terminal disulfide bridge. Biochemistry 42:11417–11426
Vadyvaloo V, Hastings JW, Van der Merwe MJ, Rautenbach M (2002) Membranes of class IIa bacteriocin-resistant Listeria monocytogenes cells contain increased levels of desaturated and short-acyl-chain phosphatidylglycerols. Appl Environ Microbiol 68:5223–5230
Vadyvaloo V, Arous S, Gravesen A, Héchard Y, Chauhan-Haubrock R, Hastings JW, Rautenbach M (2004) Cell-surface alterations in class IIa bacteriocin-resistant Listeria monocytogenes strains. Microbiology 150:3025–3033
Van Reenen CA, Dicks LMT, Chikindas ML (1998) Isolation, purification and partial characterization of plantaricin 423, a bacteriocin produced by Lactobacillus plantarum. J Appl Microbiol 84(84):1131–1137
Van Reenen CA, Chikindas ML, Van Zyl WH, Dicks LMT (2003) Characterization and heterologous expression of class IIa bacteriocin, plantaricin 423 from Lactobacillus plantarum in Saccharomyces cerevisiae. Int J Food Microbiol 81:29–40
Vaughan A, Eijsink VGH, O’Sullivan TF, O’Hanlon K, van Sinderen D (2001) An analysis of bacteriocins produced by lactic acid bacteria isolated from malted barley. J Appl Microbiol 91:131–138
Vu-Khac H, Miller KW (2009) Regulation of mannose phosphotransferase system permease and virulence gene expression in Listeria monocytogenes by the EII(t)Man transporter. Appl Environ Microbiol 75:6671–6678
Wachsman MB, Farías ME, Takeda E, Sesma F, De Ruiz Holdago A, De Torres RA, Coto CE (1999) Antiviral activity of enterocin CRL35 against herpes viruses. Int J Antimicrob Agents 12:293e299
Wachsman MB, Castilla V, Holgado APD, De Torres RA, Sesma F, Coto CE (2003) Enterocin CRL35 inhibits late stages of HSV-1 and HSV-2 replication in vitro. Antiviral Res 58:17–24
Watson RM, Woody RW, Lewis RV, Bohle DS, Andreotti AH, Ray B, Miller KW (2001) Conformational changes in pediocin AcH upon vesicle binding and approximation of the membrane-bound structure in detergent micelles. Biochemistry 40:14037–14046
Yamazaki K, Suzuki M, Kawai Y, Inoue N, Montville TJ (2005) Purification and characterization of a novel class IIa bacteriocin, piscicocin CS526, from surimi-associated Carnobacterium piscicola CS526. Appl Environ Microbiol 71:554–557
Yan LZ, Gibbs AC, Stiles ME, Wishart DS, Vederas JC (2000) Analogues of bacteriocins: antimicrobial specificity and interactions of leucocin A with its enantiomer, carnobacteriocin B2, and truncated derivatives. J Med Chem 43:4579–8451
Zhang J, Liu G, Shang N, Cheng W, Chen S, Li P (2009) Purification and partial amino acid sequence of pentocin 31-1, an anti-Listeria bacteriocin produced by Lactobacillus pentosus 31-1. J Food Prot 72:2524–529
Acknowledgments
The authors would like to thank Prof. Dzung Diep, Dr. Bruce Seal, Prof. Mike Chikindas, and Dr. Sutyak Noll for critical reading of the manuscript and stimulating discussions. YB was recipient of PhD scholarship awarded by la Région des Pays de la Loire http://www.paysdelaloire.fr/
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Belguesmia, Y., Naghmouchi, K., Chihib, NE., Drider, D. (2011). Class IIa Bacteriocins: Current Knowledge and Perspectives. In: Drider, D., Rebuffat, S. (eds) Prokaryotic Antimicrobial Peptides. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-7692-5_10
Download citation
DOI: https://doi.org/10.1007/978-1-4419-7692-5_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-7691-8
Online ISBN: 978-1-4419-7692-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)