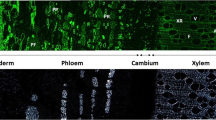
Abstract
Plant vascular pathogens use different ways to reach the xylem vessels and cause devastating diseases in plants. Resistant and tolerant plants have evolved various defense mechanisms against vascular pathogens. Inducible physico-chemical structures, such as the formation of tyloses and wall reinforcements with phenolic polymers, are very effective barriers that confine the pathogen and prevent colonization. Here, we use a combination of classical histochemistry along with bright-field and fluorescence microscopy and two-dimensional nuclear magnetic resonance (2D-NMR) spectroscopy to visualize and characterize wall reinforcements containing phenolic wall polymers, namely, lignin, ferulates, and suberin, which occur in different xylem vasculature in response to pathogen attack.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Yadeta KA, Thomma BPHJ (2013) The xylem as battleground for plant hosts and vascular wilt pathogens. Front Plant Sci 4:97
Bae C, Han SW, Song YR et al (2015) Infection processes of xylem-colonizing pathogenic bacteria: possible explanations for the scarcity of qualitative disease resistance genes against them in crops. Theor Appl Genet 128:1219–1229
Kashyap A, Planas-Marquès M, Valls M (2021) Blocking intruders: inducible physico-chemical barriers against plant vascular wilt pathogens. J Exp Bot 72:184–198
Beckman CH, Roberts EM (1995) On the nature and genetic basis for resistance and tolerance to fungal wilt diseases of plants. Adv Bot Res 21:35–77
Ferreira V, Pianzzola MJ, Vilaró FL et al (2017) Interspecific potato breeding lines display differential colonization patterns and induced defense responses after Ralstonia solanacearum infection. Front Plant Sci 8:1–14
Kashyap A, Jimenez-Jimenez AL, Zhang W et al (2022) Induced ligno-suberin vascular coating and tyramine-derived hydroxycinnamic acid amides restrict Ralstonia solanacearum colonization in resistant tomato. New Phytol 234:1411–1429
Robb J, Lee S-W, Mohan R et al (2008) Chemical characterization of stress-induced vascular coating in tomato. Plant Physiol 97:528–536
Zaini PA, Nascimento R, Gouran H et al (2018) Molecular profiling of pierce’s disease outlines the response circuitry of Vitis vinifera to Xylella fastidiosa infection. Front Plant Sci 9:771
Planas-Marquès M, Kressin JP, Kashyap A et al (2019) Four bottlenecks restrict colonization and invasion by the pathogen Ralstonia solanacearum in resistant tomato. J Exp Bot 71:2157–2171
Negrel J, Pollet B, Lapierre C (1996) Ether-linked ferulic acid amides in natural and wound periderms of potato tuber. Phytochemistry 43:1195–1199
Graça J (2010) Hydroxycinnamates in suberin formation. Phytochem Rev 9:85–91
Graça J (2015) Suberin: the biopolyester at the frontier of plants. Front Chem 3:1–11
Boher P, Serra O, Soler M et al (2013) The potato suberin feruloyl transferase FHT which accumulates in the phellogen is induced by wounding and regulated by abscisic and salicylic acids. J Exp Bot 64:3225–3236
Ralph J, Landucci L (2010) NMR of lignins. In: Heitner JA, Dimmel C, Scmidt DR (eds) Lignin and lignans: Adv chem. CRC Press, Taylor & Francis, Boca Raton, pp 137–243
Correia VG, Bento A, Pais J et al (2020) The molecular structure and multifunctionality of the cryptic plant polymer suberin. Materials Today Bio 5:100039
Campos L, Lisón P, López-Gresa MP et al (2014) Transgenic tomato plants overexpressing tyramine N-hydroxycinnamoyltransferase exhibit elevated hydroxycinnamic acid amide levels and enhanced resistance to Pseudomonas syringae. Mol Plant-Microbe Interact 27:1159–1169
Pomar F, Novo M, Bernal MA et al (2004) Changes in stem lignins (monomer composition and crosslinking) and peroxidase are related with the maintenance of leaf photosynthetic integrity during Verticillium wilt in Capsicum annuum. New Phytol 163:111–123
Harris PJ, Trethewey JAK (2010) The distribution of ester-linked ferulic acid in the cell walls of angiosperms. Phytochem Rev 9:19–33
Chang H, Cowling EB, Brown W et al (1975) Comparative studies on cellulolytic enzyme lignin and milled wood lignin of sweetgum and spruce. Holzforschung 29:153–159
Rencoret J, Kim H, Evaristo AB et al (2018) Variability in lignin composition and structure in cell walls of different parts of macaúba (Acrocomia aculeata) palm fruit. J Agric Food Chem 66:138–153
del Río JC, Rencoret J, Gutiérrez A et al (2018) Structural characterization of lignin from maize (Zea mays L.) fibers: evidence for diferuloylputrescine incorporated into the lignin polymer in maize kernels. J Agric Food Chem 66:4402–4413
Mahmoud AB, Danton O, Kaiser M et al (2020) Lignans, amides, and saponins from Haplophyllum tuberculatum and their antiprotozoal activity. Molecules 25:2825
Youngsung J, Kim H, Kang M et al (2021) Pith-specific lignification in Nicotiana atenuata as a defense against a stem-boring herbivore. New Phytol 232:332–344
Nomberg G, Marinov O, Arya GC et al (2022) The key enzymes in the suberin biosynthetic pathway in plants: an update. Plan Theory 11:392
Acknowledgments
Research was funded through MCIN/AEI/ 10.13039/501100011033 and “ERDF A way of making Europe” with grants PID2019-108595RB-I00 (NSC) and PID2020-118968RB-I00 (JR) and with the fellowship PRE2020-092086 (ALJ-J). WZ is a recipient of the China Scholarship Council fellowship (CSC NO.201906990041). Research at CRAG is also funded through the “Severo Ochoa Programme for Centres of Excellence in R&D” (CEX2019-000902-S by MCIN/AEI/10.13039/501100011033) and through the CERCA Programme/Generalitat de Catalunya.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Zhang, W., Jiménez-Jiménez, Á., Capellades, M., Rencoret, J., Kashyap, A., Coll, N.S. (2024). Determination of De Novo Suberin-Lignin Ferulate Deposition in Xylem Tissue Upon Vascular Pathogen Attack. In: Agusti, J. (eds) Xylem. Methods in Molecular Biology, vol 2722. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3477-6_9
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3477-6_9
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3476-9
Online ISBN: 978-1-0716-3477-6
eBook Packages: Springer Protocols