Abstract
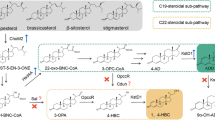
Hydroxylation of steroids has acquired special relevance for the pharmaceutical industry. Particularly, the 11α-hydroxylation of steroids is a process of biotechnological importance currently carried out at industrial scale for the production of contraceptive drugs and glucocorticoids. This process is performed by several fungal species including Rhizopus nigricans, Aspergillus ochraceus, Aspergillus niger, and Rhizopus oryzae that are used to produce by biotransformation hydroxylated steroids for pharmaceutical purposes (Wang et al., J Steroid Biochem Mol Biol 171:254–261, 2017). However, the development of more efficient biotransformation processes is essential since the steroidal derivatives obtained by the in vivo hydroxylation are often a mixture of hydroxylated compounds in different positions of the steroid molecule. This phenomenon is due to the large number of different CYPs contained in the fungal strains.
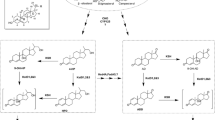
The genes responsible for the 11α-hydroxylase activity in R. oryzae consisting in the cytochrome CYP509C12 and its redox partner, the reductase RoCPR1, have been chemically synthetized forming a synthetic operon named FUN optimized to be expressed in bacteria. To express this operon, we have selected the strain Corynebacterium glutamicum R31 that is a robust and GRAS bacterial strain widely used for industrial purposes. The synthetic operon has been cloned in the pECXK-99E vector, yielding pXKFUN plasmid, and transformed C. glutamicum R31 to generate C. glutamicum R31 (pXKFUN) strain. This strain is not a steroid degrader and can efficiently transport C19 and C21 steroids across the cytoplasmic membrane (García-Fernandez et al. Catalysts 316:1–12, 2017). C. glutamicum can be used as a clean host for steroid biotransformation, because it does not introduce additional undesired side reactions on the steroids, thus reducing the contamination of the final products (Felpeto-Santero et al., Microbiol Biotechnol 12:856–868, 2019). Here we show a proof of concept that C. glutamicum can be used as a suitable chassis to perform steroid biotransformation expressing eukaryotic cytochromes. The protocol below provides detailed information on steroid 11α-hydroxylations by Corynebacterium recombinant strain.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Kollerov V, Shutov A, Fokina V et al (2010) Bioconversion of C19- and C21-steroids with parent and mutant strains of Curvularia lunata. Prikl Biokhim Mikrobiol 46:212–220
Wang R, Sui P, Hou X et al (2017) Cloning and identification of a novel steroid 11α-hydroxylase gene from Absidia coerulea. J Steroid Biochem Mol Biol 171:254–261
García-Fernandez J, Galán B, Felpeto-Santero C et al (2017) Production of 4-Ene-3-ketosteroids in Corynebacterium glutamicum. Catalysts 316:1–12
Felpeto-Santero C, Galán B, Luengo JM et al (2019) Identification and expression of the 11β-steroid hydroxylase from Cochliobolus lunatus in Corynebacterium glutamicum. Microb Biotechnol 12:856–868
Fernández-Cabezón L, Galán B, García JL (2018) New insights on steroid biotechnology. Front Microbiol 9:958
Lednicer D (2011) Steroid chemistry at a glance. Wiley, New York
Donova MV, Egorova OV (2012) Microbial steroid transformations: current state and prospects. Appl Microbiol Biotechnol 94:1423–1447
Szaleniec M, Wojtkiewicz AM, Bernhardt R et al (2018) Bacterial steroid hydroxylases: enzyme classes, their functions and comparison of their catalytic mechanisms. Appl Microbio Biotechno 102:8153–8171
Bernhardt R (2006) Cytochromes P450 as versatile biocatalysts. J Biotechnol 124:128–145
Kristan K, Rižner TL (2012) Steroid-transforming enzymes in fungi. J Steroid Biochem Mol Biol 129:79–91
Donova MV (2017) Steroid bioconversions. Methods Mol Biol 1645:1–13
Petrič Š, Hakki T, Bernhardt R et al (2010) Discovery of a steroid 11α-hydroxylase from Rhizopus oryzae and its biotechnological application. J Bacteriol 150:428–437
Fernandes P, Cruz A, Angelova B et al (2003) Microbial conversion of steroid compounds: recent developments. Enzym Microb Technol 32:688–705
Ikeda M, Nakagawa S (2003) The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl Microbiol Biotechnol 62:99–109
Felpeto-Santero C, Galán B, García JL (2021) Production of 11α-hydroxysteroids from sterols in a single fermentation step by Mycolicibacterium smegmatis. Microbl Biotechnol 14:2514–2524
Felpeto-Santero C, Thesis D (2016) Producción de esteroides mediante ingeniería de citocromos. Facultad de Ciencias Químicas, Universidad Complutense de Madrid
Kalinowski J, Bathe B, Bartels D et al (2003) The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J Biotechnol 104:5–25
De Lorenzo V (2015) Chassis organism from Corynebacterium glutamicum: the way towards biotechnological domestication of Corynebacteria. Biotechnol J 10:244–245
Santamaría RI, Gil JA, Martin JF et al (1985) High-frequency transformation of Brevibacterium lactofermentum protoplasts by plasmid DNA. J Bacteriol 162:463–467
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Galán, B., Felpeto-Santero, C., García, J.L. (2023). Production of 11α-Hydroxysteroid Derivatives by Corynebacterium glutamicum Expressing the Rhizopus oryzae Hydroxylating System. In: Barreiro, C., Barredo, JL. (eds) Microbial Steroids. Methods in Molecular Biology, vol 2704. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3385-4_17
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3385-4_17
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3384-7
Online ISBN: 978-1-0716-3385-4
eBook Packages: Springer Protocols