Abstract
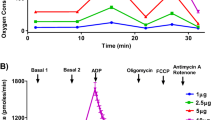
Mitochondrial respiration is an essential component of cellular metabolism. It is a process of energy conversion through enzymatically mediated reactions, the energy of taken-up substrates transformed to the ATP production. Seahorse equipment allows to measure oxygen consumption in living cells and estimate key parameters of mitochondrial respiration in real-time mode. Four key mitochondrial respiration parameters could be measured: basal respiration, ATP-production coupled respiration, maximal respiration, and proton leak. This approach demands the application of mitochondrial inhibitors—oligomycin to inhibit ATP synthase, FCCP—to uncouple the inner mitochondrial membrane and allow maximum electron flux through the electron transport chain, rotenone, and antimycin A to inhibit complexes I and III, respectively. This chapter describes two protocols of seahorse measurements performed on iPSC-derived cardiomyocytes and TAZ knock-out C2C12 cell line.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- iPSC-CM:
-
iPSC-derived cardiomyocytes
- KO:
-
Knock-out cells
- OCR:
-
Oxygen consumption rate
- WT:
-
Wild type
References
Hargreaves M, Spriet LL (2020) Skeletal muscle energy metabolism during exercise. Nat Metab 2:817–828. https://doi.org/10.1038/s42255-020-0251-4
Hood DA, Memme JM, Oliveira AN, Triolo M (2019) Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu Rev Physiol 81:19–41. https://doi.org/10.1146/annurev-physiol-020518-114310
Ignatieva E, Smolina N, Kostareva A, Dmitrieva R (2021) Skeletal muscle mitochondria dysfunction in genetic neuromuscular disorders with cardiac phenotype. Int J Mol Sci 22:7349. https://doi.org/10.3390/ijms22147349
Giacomelli E, Mummery CL, Bellin M (2017) Human heart disease: lessons from human pluripotent stem cell-derived cardiomyocytes. Cell Mol Life Sci 74:3711–3739. https://doi.org/10.1007/s00018-017-2546-5
Brodehl A, Ebbinghaus H, Deutsch MA et al (2019) Human induced pluripotent stem-cell-derived cardiomyocytes as models for genetic cardiomyopathies. Int J Mol Sci 20:4381. https://doi.org/10.3390/ijms20184381
Thomas D, Cunningham NJ, Shenoy S, Wu JC (2022) Human-induced pluripotent stem cells in cardiovascular research: current approaches in cardiac differentiation, maturation strategies, and scalable production. Cardiovasc Res 118:20–36. https://doi.org/10.1093/cvr/cvab115
Tanosaki S, Tohyama S, Kishino Y et al (2021) Metabolism of human pluripotent stem cells and differentiated cells for regenerative therapy: a focus on Cardiomyocytes. Inflamm Regen 41:5. https://doi.org/10.1186/s41232-021-00156-9
Feyen DAM, McKeithan WL, Bruyneel AAN et al (2020) Metabolic maturation media improve physiological function of human IPSC-derived cardiomyocytes. Cell Rep 32:107925. https://doi.org/10.1016/j.celrep.2020.107925
Horikoshi Y, Yan Y, Terashvili M, Wells C et al (2019) Fatty acid-treated induced pluripotent stem cell-derived human cardiomyocytes exhibit adult cardiomyocyte-like energy metabolism phenotypes. Cell 8:1095. https://doi.org/10.3390/cells8091095
Yang X, Rodriguez ML, Leonard A et al (2019) Fatty acids enhance the maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Rep 13:657–668. https://doi.org/10.1016/j.stemcr.2019.08.013
Stocco A, Smolina N, Sabatelli P et al (2021) Treatment with a triazole inhibitor of the mitochondrial permeability transition pore fully corrects the pathology of sapje zebrafish lacking dystrophin. Pharmacol Res 165:105421. https://doi.org/10.1016/j.phrs.2021.105421
Lian X, Hsiao C, Wilson G et al (2012) Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A 109. https://doi.org/10.1073/pnas.1200250109
Tohyama S, Hattori F, Sano M et al (2013) Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12:127–137. https://doi.org/10.1016/j.stem.2012.09.013
Acknowledgments
This work was supported by Russian Scientific Foundation grant number 20-15-00271.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Smolina, N., Khudiakov, A., Kostareva, A. (2023). Assaying Mitochondrial Respiration as an Indicator of Cellular Metabolism and Fitness. In: Friedrich, O., Gilbert, D.F. (eds) Cell Viability Assays. Methods in Molecular Biology, vol 2644. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3052-5_1
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3052-5_1
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3051-8
Online ISBN: 978-1-0716-3052-5
eBook Packages: Springer Protocols