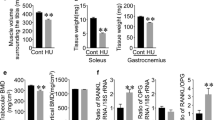
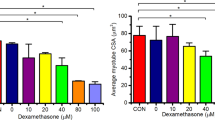
Abstract
Skeletal muscle is a highly plastic tissue that can alter its mass and strength in response to mechanical stimulation, such as overloading and unloading, which lead to muscle hypertrophy and atrophy, respectively. Mechanical loading in the muscle influences muscle stem cell dynamics, including activation, proliferation, and differentiation. Although experimental models of mechanical overloading and unloading have been widely used for the investigation of the molecular mechanisms regulating muscle plasticity and stem cell function, few studies have described the methods in detail. Here, we describe the appropriate procedures for tenotomy-induced mechanical overloading and tail-suspension-induced mechanical unloading, which are the most common and simple methods to induce muscle hypertrophy and atrophy in mouse models.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Janssen I, Heymsfield SB, Wang ZM et al (2000) Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol 89(1):81–88
Baskin KK, Winders BR, Olson EN (2015) Muscle as a “mediator” of systemic metabolism. Cell Metab 21:237–248
Miyazaki M, Esser KA (2009) Cellular mechanisms regulating protein synthesis and skeletal muscle hypertrophy in animals. J Appl Physiol 106(4):1367–1373
Young A, Stokes M, Round JM et al (1983) The effect of high-resistance training on the strength and cross-sectional area of the human quadriceps. Eur J Clin Investig 13(5):411–417
Kandarian SC, Young JC, Gomez EE (1992) Adaptation in synergistic muscles to soleus and plantaris muscle removal in the rat hindlimb. Life Sci 51(21):1691–1698
LeBlanc A, Gogia P, Schneider V et al (1988) Calf muscle area and strength changes after five weeks of horizontal bed rest. Am J Sports Med 16(6):624–629
Cadena SM, Zhang Y, Fang J et al (2019) Skeletal muscle in MuRF1 null mice is not spared in low-gravity conditions, indicating atrophy proceeds by unique mechanisms in space. Sci Rep 9(1):9397
Zammit PS, Golding JP, Nagata Y et al (2004) Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol 166(3):347–357
Fujimaki S, Hidaka R, Asashima M et al (2014) Wnt protein-mediated satellite cell conversion in adult and aged mice following voluntary wheel running. J Biol Chem 289(11):7399–7412
Fujimaki S, Wakabayashi T, Asashima M et al (2016) Treadmill running induces satellite cell activation in diabetic mice. Biochem Biophys Rep 8:6–13
Fujimaki S, Machida M, Wakabayashi T et al (2016) Functional overload enhances satellite cell properties in skeletal muscle. Stem Cells Int 2016:7619418
Fukuda S, Kaneshige A, Kaji T et al (2019) Sustained expression of HeyL is critical for the proliferation of muscle stem cells in overloaded muscle. elife 8:e48284
Guerci A, Lahoute C, Hébrard S et al (2012) Srf-dependent paracrine signals produced by myofibers control satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 15(1):25–37
Guo BS, Cheung KK, Yeung SS et al (2012) Electrical stimulation influences satellite cell proliferation and apoptosis in unloading-induced muscle atrophy in mice. PLoS One 7(1):e30348
Arentson-Lantz EJ, English KL, Paddon-Jones D et al (2016) Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol 120(8):965–975
Guitart M, Lloreta J, Mañas-Garcia L et al (2018) Muscle regeneration potential and satellite cell activation profile during recovery following hindlimb immobilization in mice. J Cell Physiol 233(5):4360–4372
Brooks MJ, Hajira A, Mohamed JS et al (2018) Voluntary wheel running increases satellite cell abundance and improves recovery from disuse in gastrocnemius muscles from mice. J Appl Physiol 124(6):1616–1628
Phelan JN, Gonyea WJ (1997) Effect of radiation on satellite cell activity and protein expression in overloaded mammalian skeletal muscle. Anat Rec 247(2):179–188
Fukada SI, Akimoto T, Sotiropoulos A (2020) Role of damage and management in muscle hypertrophy: different behaviors of muscle stem cells in regeneration and hypertrophy. Biochim Biophys Acta, Mol Cell Res 1867(9):118742
Hubbard RW, Ianuzzo CD, Mathew WT et al (1975) Compensatory adaptations of skeletal muscle composition to a long-term functional overload. Growth 39(1):85–93
Fry CS, Lee JD, Jackson JR et al (2014) Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28(4):1654–1665
Hanson ED, Betik AC, Timpani CA et al (2020) Testosterone suppression does not exacerbate disuse atrophy and impairs muscle recovery that is not rescued by high protein. J Appl Physiol 129(1):5–16
Brocca L, Toniolo L, Reggiani C et al (2017) FoxO-dependent atrogenes vary among catabolic conditions and play a key role in muscle atrophy induced by hindlimb suspension. J Physiol 595(4):1143–1158
Booth FW (1978) Regrowth of atrophied skeletal muscle in adult rats after ending immobilization. J Appl Physiol Respir Environ Exerc Physiol 44(2):225–230
Fujimaki S, Matsumoto T, Muramatsu M et al (2022) The endothelial Dll4–muscular Notch2 axis regulates skeletal muscle mass. Nat Metab 4(2):180–189
Acknowledgments
This work was supported by the Japan Agency for Medical Research and Development (AMED, JP18ek0109383 and JP19bm0704036), the FOREST program of the Japan Science and Technology Agency (JST, JPMJFR205C) the Grant-in-Aid for Scientific Research KAKENHI (18H03193, 20K21763, 20K19641, 22K18414 and 22H00505) from the Japan Society for the Promotion of Science (JSPS), the Uehara Memorial Foundation, and the Takeda Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Fujimaki, S., Ono, Y. (2023). Murine Models of Tenotomy-Induced Mechanical Overloading and Tail-Suspension-Induced Mechanical Unloading. In: Asakura, A. (eds) Skeletal Muscle Stem Cells. Methods in Molecular Biology, vol 2640. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3036-5_15
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3036-5_15
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3035-8
Online ISBN: 978-1-0716-3036-5
eBook Packages: Springer Protocols