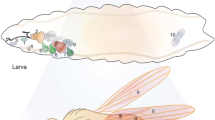
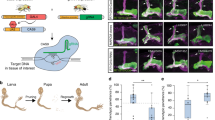
Abstract
Genetic ablation is a highly efficient method to study regeneration in vivo by stimulating tissue-specific cell death that subsequently induces regrowth and repair in a develo** organism. This approach has been particularly successful in Drosophila, for which various temperature-based genetic ablation tools have been developed to explore the complexities of regeneration in larval imaginal discs. Here, we describe the use of a recently established ablation system called DUAL Control, which can be used to both characterize the damage response and genetically manipulate blastema cells to identify novel regulators of regeneration.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Morgan TH (1901) Regeneration and liability to injury. Science 14(346):235–248. https://doi.org/10.1126/science.14.346.235
Li Q, Yang H, Zhong TP (2015) Regeneration across metazoan phylogeny: lessons from model organisms. J Genet Genomics 42(2):57–70. https://doi.org/10.1016/j.jgg.2014.12.002
Fox DT, Cohen E, Smith-Bolton R (2020) Model systems for regeneration: Drosophila. Development 147(7). https://doi.org/10.1242/dev.173781
Marques IJ, Lupi E, Mercader N (2019) Model systems for regeneration: zebrafish. Development 146(18). https://doi.org/10.1242/dev.167692
Worley MI, Setiawan L, Hariharan IK (2012) Regeneration and transdetermination in Drosophila imaginal discs. Annu Rev Genet 46:289–310. https://doi.org/10.1146/annurev-genet-110711-155637
Smith-Bolton R (2016) Drosophila imaginal discs as a model of epithelial wound repair and regeneration. Adv Wound Care (New Rochelle) 5(6):251–261. https://doi.org/10.1089/wound.2014.0547
Hariharan IK, Serras F (2017) Imaginal disc regeneration takes flight. Curr Opin Cell Biol 48:10–16. https://doi.org/10.1016/j.ceb.2017.03.005
Bergantinos C, Vilana X, Corominas M, Serras F (2010) Imaginal discs: renaissance of a model for regenerative biology. BioEssays 32(3):207–217. https://doi.org/10.1002/bies.200900105
Beira JV, Paro R (2016) The legacy of Drosophila imaginal discs. Chromosoma 125(4):573–592. https://doi.org/10.1007/s00412-016-0595-4
Bryant PJ (1971) Regeneration and duplication following operations in situ on the imaginal discs of Drosophila melanogaster. Dev Biol 26(4):637–651. https://doi.org/10.1016/0012-1606(71)90146-1
Hadorn E, Hurlimann R, Mindek G, Schubiger G, Staub M (1968) Developmental capacity of embryonal blastema in Drosophila following cultivation in an adult host. Rev Suisse Zool 75(3):557–569
Haynie JL, Bryant PJ (1977) The effects of X-rays on the proliferation dynamics of cells in the imaginal wing disc of Drosophila melanogaster. Wilehm Roux Arch Dev Biol 183(2):85–100. https://doi.org/10.1007/BF00848779
Schubiger G (1971) Regeneration, duplication and transdetermination in fragments of the leg disc of Drosophila melanogaster. Dev Biol 26(2):277–295. https://doi.org/10.1016/0012-1606(71)90127-8
Sweeney ST, Hidalgo A, de Belle JS, Keshishian H (2012) Genetic systems for functional cell ablation in Drosophila. Cold Spring Harb Protoc 2012(9):950–956. https://doi.org/10.1101/pdb.top068361
Smith-Bolton RK, Worley MI, Kanda H, Hariharan IK (2009) Regenerative growth in Drosophila imaginal discs is regulated by wingless and Myc. Dev Cell 16(6):797–809. https://doi.org/10.1016/j.devcel.2009.04.015
Bergantinos C, Corominas M, Serras F (2010) Cell death-induced regeneration in wing imaginal discs requires JNK signalling. Development 137(7):1169–1179. https://doi.org/10.1242/dev.045559
Harris RE, Stinchfield MJ, Nystrom SL, McKay DJ, Hariharan IK (2020) Damage-responsive, maturity-silenced enhancers regulate multiple genes that direct regeneration in Drosophila. elife 9. https://doi.org/10.7554/eLife.58305
Herrera SC, Martin R, Morata G (2013) Tissue homeostasis in the wing disc of Drosophila melanogaster: immediate response to massive damage during development. PLoS Genet 9(4):e1003446. https://doi.org/10.1371/journal.pgen.1003446
Herrera SC, Morata G (2014) Transgressions of compartment boundaries and cell reprogramming during regeneration in Drosophila. elife 3:e01831. https://doi.org/10.7554/eLife.01831
Repiso A, Bergantinos C, Serras F (2013) Cell fate respecification and cell division orientation drive intercalary regeneration in Drosophila wing discs. Development 140(17):3541–3551. https://doi.org/10.1242/dev.095760
Vizcaya-Molina E, Klein CC, Serras F, Mishra RK, Guigo R, Corominas M (2018) Damage-responsive elements in Drosophila regeneration. Genome Res 28(12):1852–1866. https://doi.org/10.1101/gr.233098.117
Santabarbara-Ruiz P, Esteban-Collado J, Perez L, Viola G, Abril JF, Milan M, Corominas M, Serras F (2019) Ask1 and Akt act synergistically to promote ROS-dependent regeneration in Drosophila. PLoS Genet 15(1):e1007926. https://doi.org/10.1371/journal.pgen.1007926
Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM (2010) Refinement of tools for targeted gene expression in Drosophila. Genetics 186(2):735–755. https://doi.org/10.1534/genetics.110.119917
Lai SL, Lee T (2006) Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci 9(5):703–709. https://doi.org/10.1038/nn1681
Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118(2):401–415
Blair SS (2007) Dissection of imaginal discs in Drosophila. CSH Protoc 2007:pdb prot4794. https://doi.org/10.1101/pdb.prot4794
Acknowledgments
This work was supported by a grant from the NICHD R21 HD102765-01 to R.E.H.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Harris, R.E. (2023). Investigating Tissue Regeneration Using the DUAL Control Genetic Ablation System. In: Simoes-Costa, M. (eds) DNA-Protein Interactions. Methods in Molecular Biology, vol 2599. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2847-8_18
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2847-8_18
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2846-1
Online ISBN: 978-1-0716-2847-8
eBook Packages: Springer Protocols