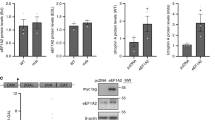
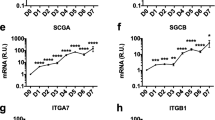
Abstract
Duchenne muscular dystrophy (DMD) is a neuromuscular disease caused by mutations and deletions within the DMD gene, which result in a lack of dystrophin protein at the sarcolemma of skeletal muscle fibers. The absence of dystrophin fragilizes the sarcolemma and compromises its integrity during cycles of muscle contraction, which, progressively, leads to reductions in muscle mass and function. DMD is thus a progressive muscle-wasting disease that results in a loss of ambulation, cardiomyopathy, respiratory impairment, and death. Although there is presently no cure for DMD, recent advances have led to many promising treatments. One such approach entails increasing expression of a homologous protein to dystrophin, named utrophin A, which is endogenously expressed in both healthy and DMD muscle fibers. Upregulation of utrophin A all along the sarcolemma of DMD muscle fibers can, in part, compensate for the absence of dystrophin. Over the years, our laboratory has focused a significant portion of our efforts in identifying and characterizing drugs and small molecules for their ability to target utrophin A and cause its overexpression. As part of these efforts, we have recently developed a novel ELISA-based high-throughput drug screen, to identify FDA-approved drugs that increase the expression of utrophin A in muscle cells in culture as well as in dystrophic mice. Here, we describe our overall strategy to identify and characterize several FDA-approved drugs that upregulate utrophin A expression and provide details on all experimental approaches. Such strategy has the potential to lead to the rapid development of novel therapeutics for DMD.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Baby SM, Bogdanovich S, Willmann G et al (2010) Differential expression of utrophin-A and -B promoters in the central nervous system (CNS) of normal and dystrophic mdx mice. Brain Pathol 20:323–342
Burton EA, Tinsley JM, Holzfeind PJ et al (1999) A second promoter provides an alternative target for therapeutic up-regulation of utrophin in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 96:14025–14030
Dennis CL, Tinsley JM, Deconinck AE et al (1996) Molecular and functional analysis of the utrophin promoter. Nucleic Acids Res 24:1646–1652
Nguyen TM, Ellis JM, Love DR et al (1991) Localization of the DMDL gene-encoded dystrophin-related protein using a panel of nineteen monoclonal antibodies: presence at neuromuscular junctions, in the sarcolemma of dystrophic skeletal muscle, in vascular and other smooth muscles, and in proliferating brain cell lines. J Cell Biol 115:1695–1700
Weir AP, Burton EA, Harrod G et al (2002) A- and B-utrophin have different expression patterns and are differentially up-regulated in mdx muscle. J Biol Chem 277:45285–45290
Nakamura A, Takeda S. Mammalian models of Duchenne muscular dystrophy: pathological characteristics and therapeutic applications. https://www.hindawi.com/journals/bmri/2011/184393/
Goyenvalle A, Seto JT, Davies KE et al (2011) Therapeutic approaches to muscular dystrophy. Hum Mol Genet 20:R69–R78
Durbeej M, Campbell KP (2002) Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev 12:349–361
Chakkalakal JV, Thompson J, Parks RJ et al (2005) Molecular, cellular, and pharmacological therapies for Duchenne/Becker muscular dystrophies. FASEB J 19:880–891
Bogdanovich S, Perkins KJ, Krag TOB et al (2004) Therapeutics for Duchenne muscular dystrophy: current approaches and future directions. J Mol Med 82:102–115
Gregorevic P, Blankinship MJ, Allen JM et al (2004) Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 10:828–834
Gregorevic P, Blankinship MJ, Allen JM et al (2008) Systemic microdystrophin gene delivery improves skeletal muscle structure and function in old dystrophic mdx mice. Mol Ther 16:657–664
Rodino-Klapac LR, Janssen PML, Shontz KM et al (2013) Micro-dystrophin and follistatin co-delivery restores muscle function in aged DMD model. Hum Mol Genet 22:4929–4937
Guiraud S, Davies KE (2017) Pharmacological advances for treatment in Duchenne muscular dystrophy. Curr Opin Pharmacol 34:36–48
Ramos J, Chamberlain JS (2015) Gene therapy for Duchenne muscular dystrophy. Expert Opin Orphan Drugs 3:1255–1266
Odom GL, Gregorevic P, Allen JM et al (2008) Microutrophin delivery through rAAV6 increases lifespan and improves muscle function in dystrophic dystrophin/utrophin-deficient mice. Mol Ther 16:1539–1545
Guiraud S, Squire SE, Edwards B et al (2015) Second-generation compound for the modulation of utrophin in the therapy of DMD. Hum Mol Genet 24:4212–4224
Tinsley JM, Fairclough RJ, Storer R et al (2011) Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PLoS One 6:e19189
Squire S, Raymackers JM, Vandebrouck C et al (2002) Prevention of pathology in mdx mice by expression of utrophin: analysis using an inducible transgenic expression system. Hum Mol Genet 11:3333–3344
Tinsley J, Deconinck N, Fisher R et al (1998) Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med 4:1441–1444
Péladeau C, Adam NJ, Jasmin BJ (2018) Celecoxib treatment improves muscle function in mdx mice and increases utrophin A expression. FASEB J 32:5090–5103
Péladeau C, Ahmed A, Amirouche A et al (2016) Combinatorial therapeutic activation with heparin and AICAR stimulates additive effects on utrophin A expression in dystrophic muscles. Hum Mol Genet 25:24–43
Péladeau C, Adam N, Bronicki LM et al (2020) Identification of therapeutics that target eEF1A2 and upregulate utrophin A translation in dystrophic muscles. Nat Commun 11:1990
Al-Rewashdy H, Ljubicic V, Lin W et al (2015) Utrophin A is essential in mediating the functional adaptations of mdx mouse muscle following chronic AMPK activation. Hum Mol Genet 24:1243–1255
Ljubicic V, Miura P, Burt M et al (2011) Chronic AMPK activation evokes the slow, oxidative myogenic program and triggers beneficial adaptations in mdx mouse skeletal muscle. Hum Mol Genet 20:3478–3493
Ljubicic V, Burt M, Lunde JA et al (2014) Resveratrol induces expression of the slow, oxidative phenotype in mdx mouse muscle together with enhanced activity of the SIRT1-PGC-1α axis. Am J Physiol Cell Physiol 307:C66–C82
Miura P, Chakkalakal JV, Boudreault L et al (2009) Pharmacological activation of PPARβ/δ stimulates utrophin A expression in skeletal muscle fibers and restores sarcolemmal integrity in mature mdx mice. Hum Mol Genet 18:4640–4649
Amirouche A, Tadesse H, Lunde JA et al (2013) Activation of p38 signaling increases utrophin A expression in skeletal muscle via the RNA-binding protein KSRP and inhibition of AU-rich element-mediated mRNA decay: implications for novel DMD therapeutics. Hum Mol Genet 22:3093–3111
Gordon BS, Delgado Díaz DC, Kostek MC (2013) Resveratrol decreases inflammation and increases utrophin gene expression in the mdx mouse model of duchenne muscular dystrophy. Clin Nutr 32:104–111
Vianello S, Yu H, Voisin V et al (2013) Arginine butyrate: a therapeutic candidate for Duchenne muscular dystrophy. FASEB J 27:2256–2269
Wishart DS, Feunang YD, Guo AC et al (2018) DrugBank 5.0: a major update to the DrugBank database for 2018. 46:D1074–D1082
Jahnke VE, Van Der Meulen JH, Johnston HK et al (2012) Metabolic remodeling agents show beneficial effects in the dystrophin-deficient mdx mouse model. 2:16
Ljubicic V, Jasmin BJ (2015) Metformin increases peroxisome proliferator-activated receptor γ Co-activator-1α and utrophin a expression in dystrophic skeletal muscle. Muscle Nerve 52:139–142
Charan J, Kantharia ND (2013) How to calculate sample size in animal studies? J Pharmacol Pharmacother 4:303–306
Love DR, Hill DF, Dickson G et al (1989) An autosomal transcript in skeletal muscle with homology to dystrophin. 339:55–58
McGreevy JW, Hakim CH, McIntosh MA et al (2015) Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Dis Model Mech 8:195–213
Acknowledgments
We are indebted to many current and previous members of the Jasmin lab who have made important contributions in this research program, leading us to perform this chemical screen. We would like to give special thanks to Nadine Adam for her contribution in editing the manuscript. We are also grateful to the Canadian Institutes of Health Research, the Association Française contre les Myopathies, the Muscular Dystrophy Association (USA), and Jesse’s Journey for their generous financial support over the years.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Péladeau, C., Jasmin, B.J. (2023). Identifying FDA-Approved Drugs that Upregulate Utrophin A as a Therapeutic Strategy for Duchenne Muscular Dystrophy. In: Maruyama, R., Yokota, T. (eds) Muscular Dystrophy Therapeutics. Methods in Molecular Biology, vol 2587. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2772-3_26
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2772-3_26
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2771-6
Online ISBN: 978-1-0716-2772-3
eBook Packages: Springer Protocols