Abstract
Immunohistochemistry is a valuable tool for probing not only scientific questions but also clinical diagnoses. It provides power from localization of a protein within the milieu of a tissue section that may reflect positioning within or beyond the boundaries of a cell that is representative of the tissue at a discrete moment in time. The method can be applied broadly, including to tissues under normal, developmental, chemically, or genetically altered conditions and disease states.
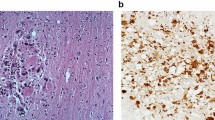
Disease manifesting from West Nile virus infection ranges from acute, systemic febrile symptoms to compromise of central nervous system function. Immunohistochemistry has been used to assess WNV infection in the nervous system in postmortem and experimental conditions, despite the lack of understanding of the precise route of viral entry. In addition to imprecise knowledge of initial viral entry into cells and whether entry is even the same between cell types, the fact that spontaneous viral mutations and environmental pressures from climate change may alter the prevalence of the disease state across geographical and climatological boundaries highlights the need for continued assessment of infection. Immunohistochemistry is a useful way to assess these aspects of WNV infection with the aim being to better understand the organs and cell types that are compromised by WNV infection. This chapter outlines how this can be carried out on brain tissue, but the procedures discussed can also be applied more broadly on tissue outside of the central nervous system.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Yee KT, Neupane B, Bai F et al (2020) Zika virus infection causes widespread damage to the inner ear. Hear Res 395:108000. https://doi.org/10.1016/j.heares.2020.108000
Yee KT, Simon HH, Tessier-Lavigne M et al (1999) Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron 24(3):607–622
Vetter DE, Adams JC, Mugnaini E (1991) Chemically distinct rat olivocochlear neurons. Synapse 7(1):21–43
Murthy V, Taranda J, Elgoyhen AB et al (2009) Activity of nAChRs containing alpha9 subunits modulates synapse stabilization via bidirectional signaling programs. Dev Neurobiol 69(14):931–949. https://doi.org/10.1002/dneu.20753
Murthy V, Maison SF, Taranda J et al (2009) SK2 channels are required for function and long-term survival of efferent synapses on mammalian outer hair cells. Mol Cell Neurosci 40(1):39–49. https://doi.org/10.1016/j.mcn.2008.08.011
Yee KT, Smetanka AM, Lund RD et al (1990) Differential expression of class I and class II major histocompatibility complex antigen in early postnatal rats. Brain Res 530(1):121–125. https://doi.org/10.1016/0006-8993(90)90667-z
Idikio HA (2009) Immunohistochemistry in diagnostic surgical pathology: contributions of protein life-cycle, use of evidence-based methods and data normalization on interpretation of immunohistochemical stains. Int J Clin Exp Pathol 3(2):169–176
von Behring E, Kitasato S (1890) Ueber das Zustandekommen der Diphtherie-Immunitata und der Tetanus-Immunitat bei Thieren. Deutsch Med Wochenschr 16:1113–1114
Heidelberger M, Kendall FE (1933) Studies on the precipitin reaction: precipitating haptens; species differences in antibodies. J Exp Med 57(3):373–379. https://doi.org/10.1084/jem.57.3.373
Marrack JR (1934) Derived antigens as a means of studying the relation of specific combination to chemical structure: (section of therapeutics and pharmacology). Proc R Soc Med 27(8):1063–1065
Coons A, Creech HJ, Jones RN (1941) Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med 47:200–202
Nakane PK, Pierce GB Jr (1966) Enzyme-labeled antibodies: preparation and application for the localization of antigens. J Histochem Cytochem 14(12):929–931. https://doi.org/10.1177/14.12.929
Childs GV (2014) History of immunohistochemistry. In: Pathology of human disease. Academic Press, Amsterdam, pp 3775–3796
Vetter DE, Cozzari C, Hartman BK et al (1993) Choline acetyltransferase in the rat cochlear nuclei: immunolocalization with a monoclonal antibody. In: Merchan MA, Juiz JM, Godfrey DA, Mugnaini E (eds) The mammalian cochlear nuclei: organization and function, NATO ASI series a: life sciences, vol 239. Plenum Press, New York, pp 279–290
Oertel WH, Schmechel DE, Mugnaini E et al (1981) Immunocytochemical localization of glutamate decarboxylase in rat cerebellum with a new antiserum. Neuroscience 6(12):2715–2735
Hsu SM, Raine L (1982) Versatility of biotin-labeled lectins and avidin-biotin-peroxidase complex for localization of carbohydrate in tissue sections. J Histochem Cytochem 30(2):157–161. https://doi.org/10.1177/30.2.7037937
Vetter DE, Liberman MC, Mann J et al (1999) Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 23(1):93–103
Adams JC (1981) Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem 29(6):775–775. https://doi.org/10.1177/29.6.7252134
Adams JC (1992) Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem 40(10):1457–1463. https://doi.org/10.1177/40.10.1527370
Smithburn KC, Hughes TP, Burke AW et al (1940) A neurotropic virus isolated from the blood of a native of Uganda 1. Am J Trop Med Hyg s1-20(4):471–492. https://doi.org/10.4269/ajtmh.1940.s1-20.471
Hofmeister EK (2011) West Nile virus: North American experience. Integrative Zoology. https://doi.org/10.1111/j.1749-4877.2011.00251.x
Turell MJ, O’Guinn ML, Dohm DJ et al (2002) Vector competence of Culex tarsalis from Orange County, California, for West Nile virus. Vector Borne Zoonotic Dis https://doi.org/10.1089/15303660260613756
Bender K, Thompson FE (2003) West Nile Virus: A growing challenge. Am J Nurs 103(6):32–39. https://doi.org/10.1097/00000446-200306000-00018
Centers for Disease Control and Prevention (2003) Detection of West Nile virus in blood donations–United States, 2003. Morb Mortal Wkly Rep 52(32):769–772
Pealer LN, Marfin AA, Petersen LR et al (2003) Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med 349(13):1236–1245. https://doi.org/10.1056/NEJMoa030969
Iwamoto M, Jernigan DB, Guasch A et al (2003) Transmission of West Nile Virus from an Organ Donor to Four Transplant Recipients. N Engl J Med 348(22):2196–2203. https://doi.org/10.1056/NEJMoa022987
Centers for Disease Control and Prevention (2002) Intrauterine West Nile virus infection–New York, 2002. Morb Mortal Wkly Rep 51(50):1135–1136
MMWR (2002) Possible West Nile virus transmission to an infant through breast-feeding—Michigan, 2002. Morb Mortal Wkly Rep 2002 515:877–878
Petersen LR, Brault AC, Nasci RS (2013) West Nile virus: Review of the literature. JAMA 310(3):308. https://doi.org/10.1001/jama.2013.8042
Bernard KA, Maffei JG, Jones SA et al (2001) West nile virus infection in birds and mosquitoes New York state 2000. Emerg Infect Dis 7(4):679–685. https://doi.org/10.3201/eid0704.010415
Oliveri RL, Jeter WC, Stark LM et al (2003) Surveillance results from the first West Nile virus transmission season in Florida 2001. Am J Trop Med Hyg 69(2):141–150. https://doi.org/10.4269/ajtmh.2003.69.141
Goddard LB, Roth AE, Reisen WK et al (2002) Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis 8(12):1385–1391. https://doi.org/10.3201/eid0812.020536
Blitvich BJ (2008) Transmission dynamics and changing epidemiology of West Nile virus. Anim Health Res Rev 9(1):71–86. https://doi.org/10.1017/S1466252307001430
Turell MJ, Dohm DJ, Sardelis MR et al (2005) An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol 42(1):57–62. https://doi.org/10.1093/jmedent/42.1.57
Turell MJ, O’Guinn ML, Dohm DJ et al (2001) Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol 38(2):130–134. https://doi.org/10.1603/0022-2585-38.2.130
Apperson CS, Hassan HK, Harrison BA et al (2004) Host feeding patterns of established and potential mosquito vectors of West Nile virus in the Eastern United States. Vector Borne Zoonotic Dis 4(1):71–82. https://doi.org/10.1089/153036604773083013
Hamer GL, Kitron UD, Brawn JD et al (2008) Culex pipiens (Diptera: Culicidae): A bridge vector of West Nile virus to humans. J Med Entomol 45(1):125–128. https://doi.org/10.1603/0022-2585(2008)45[125:CPDCAB]2.0.CO;2
Nasci RS, Savage HM, White DJ et al (2001) West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis 7(4):742–744. https://doi.org/10.3201/eid0704.010426
Komar N, Burns J, Dean C et al (2001) Serologic evidence for West Nile virus infection in birds in Staten island New York after an outbreak in 2000. Vector Borne Zoonotic Dis 1(3):191–196. https://doi.org/10.1089/153036601753552558
Komar N, Panella NA, Burns JE et al (2001) Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg Infect Dis 7(4):621–625. https://doi.org/10.3201/eid0704.010403
Komar N, Owen JC, Edwards E et al (2005) Avian hosts for West Nile virus in st. tammany Parish Louisiana 2002. Am J Trop Med Hyg 73(6):1031–1037. https://doi.org/10.4269/ajtmh.2005.73.1031
Kilpatrick AM, Kramer LD, Jones MJ et al (2006) West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol 4(4):e82. https://doi.org/10.1371/journal.pbio.0040082
Tempelis C, Reeves W, Bellamy R et al (1965) A three-year study of the feeding habits of Culex Tarsalis in Kern County, California. Am J Trop Med Hyg 14(1):170–177. https://doi.org/10.4269/ajtmh.1965.14.170
Francy DB, Tempelis CH, Hayes RO (1967) Variations in feeding patterns of seven culicine mosquitoes on vertebrate hosts in weld and larimer counties Colorado. Am J Trop Med Hyg 16(1):111–119. https://doi.org/10.4269/ajtmh.1967.16.111
Molaei G, Andreadis TG, Armstrong PM (2006) Host feeding patterns of Culex mosquitoes and West Nile virus transmission, Northeastern United States. Emerg Infect Dis 12(3):468–474. https://doi.org/10.3201/eid1203.051004
Campbell GL, Marfin AA, Lanciotti RS et al (2002) West Nile virus. Lancet Infect Dis 2(9):519–529. S1473309902003687. https://doi.org/10.1016/S1473-3099(02)00368-7
Petersen LR, Roehrig RT (2001) West Nile virus: A reemerging global pathogen. Emerg Infect Dis 7(4):611–614. https://doi.org/10.3201/eid0704.010401
Zeller HG, Schuffenecker I (2004) West Nile virus: An overview of its spread in Europe and the mediterranean basin in contrast to its spread in the Americas. Eur J Clin Microbiol Infect Dis 23(3):147–156. https://doi.org/10.1007/s10096-003-1085-1
Chowers MY, Lang R, Nassar F et al (2001) Clinical characteristics of the West Nile fever outbreak Israel 2000. Emerg Infect Dis 7(4):675–678 https://doi.org/10.3201/eid0704.010414
Tsai TF, Popovici F, Cernescu C (1998) West Nile encephalitis epidemic in southeastern Romania. Lancet 352(9130):767–771. S0140673698035387. https://doi.org/10.1016/S0140-6736(98)03538-7
McIntosh BM, Jupp PG, dos Santos I et al (1976) Epidemics of West Nile and Sindbis viruses in South Africa with Culex (Culex) univittatus Thoebald as vector. S Afri J Sci 72:295–300
Parks JJ, Ganaway JR, Price WH (1958) Studies on immunologic overlap among certain arthropod-borne viruses. III. A laboratory analysis of three strains of West Nile virus which have been studied in human cancer patients. Am J Hyg 68(2):106–119
Nir Y, Goldwasser R, Lasowski Y et al (1968) Isolation of West Nile virus strains from mosquitoes in Israel. Am J Epidemiol 87(2):496–501. https://doi.org/10.1093/oxfordjournals.aje.a120839
Savage HM, Ceianu C, Nicolescu G et al (1999) Entomologic and avian investigations of an epidemic of West Nile fever in Romania in 1996 with serologic and molecular characterization of a virus isolate from mosquitoes. Am J Trop Med Hyg 61(4):600–611. https://doi.org/10.4269/ajtmh.1999.61.600
Lanciotti RS, Ebel GD, Deubel V et al (2002) Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States Europe and the Middle East. Virology 298(1):96–105. S0042682202914492. https://doi.org/10.1006/viro.2002.1449
Danis K, Papa A, Theocharopoulos G et al (2011) Outbreak of West Nile virus infection in Greece 2010. Emerg Infect Dis 17(10):1868–1872. https://doi.org/10.3201/eid1710.110525
Papa A, Xanthopoulou K, Gewehr S et al (2011) Detection of West Nile virus lineage 2 in mosquitoes during a human outbreak in Greece. Clin Microbiol Infect 17(8):1176–1180. S1198743X14629487. https://doi.org/10.1111/j.1469-0691.2010.03438.x
Danis K, Papa A, Papanikolaou E et al (2011) Ongoing outbreak of West Nile virus infection in humans, Greece, July to August 2011. Euro Surveill 16(34):19951. https://doi.org/10.2807/ese.16.34.19951-en
Anastasiadou A, Economopoulou A, Kakoulidis I et al (2011) Non-neuroinvasive West Nile virus infections during the outbreak in Greece. Clin Microbiol Infect 17(11):1681–1683. S1198743X14618978. https://doi.org/10.1111/j.1469-0691.2011.03642.x
Fratkin JD, Leis AA, Stokic DS et al (2004) Spinal cord neuropathology in human West Nile virus infection. Arch Pathol Lab Med 128(5):533–537. https://doi.org/10.5858/2004-128-533-SCNIHW
Bai F, Thompson EA, Vig PJS et al (2019) Current understanding of West Nile virus clinical manifestations immune responses neuroinvasion and immunotherapeutic implications. Pathogens 8(4):193. https://doi.org/10.3390/pathogens8040193
Acknowledgments
The authors thank Fengwei Bai and Farzana Nazneen at the University of Southern Mississippi for providing West Nile virus-infected tissue. This work was supported by the American Otological Society (DEV, KTY), the American Hearing Research Foundation (KTY) and the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM121334 (KTY). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Yee, K.T., Vetter, D.E. (2023). Detection of West Nile Virus Envelope Protein in Brain Tissue with an Immunohistochemical Assay. In: Bai, F. (eds) West Nile Virus. Methods in Molecular Biology, vol 2585. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2760-0_7
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2760-0_7
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2759-4
Online ISBN: 978-1-0716-2760-0
eBook Packages: Springer Protocols