Abstract
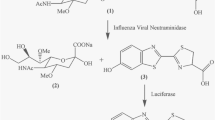
Three types of assays––colorimetric, fluorescent, and chemiluminescent––are used to determine the sialidase (neuraminidase: NA) activity of influenza viruses. The fluorescent assay is cost-effective and applicable for many laboratories and is, therefore, commonly used for global monitoring of the NA inhibitor susceptibility of influenza viruses. Here, I describe, in detail, protocols for the fluorescence-based NA activity assay and the NA inhibition assay, which are used to determine the NA activity and NA inhibitor susceptibility, respectively, of influenza viruses.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Nguyen HT, Sheu TG, Mishin VP et al (2010) Assessment of pandemic and seasonal influenza A (H1N1) virus susceptibility to neuraminidase inhibitors in three enzyme activity inhibition assays. Antimicrob Agents Chemother 54(9):3671–3677. https://doi.org/10.1128/AAC.00581-10
World Health Organization. Laboratory methodologies for testing the antiviral susceptibility of influenza viruses. https://www.who.int/teams/global-influenza-programme/laboratory-network/quality-assurance/antiviral-susceptibility-influenza. Accessed 4 July 2022
Meijer A, Rebelo-de-Andrade H, Correia V et al (2014) Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2012-2013. Antivir Res 110:31–41. https://doi.org/10.1016/j.antiviral.2014.07.001
Takashita E, Meijer A, Lackenby A et al (2015) Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2013-2014. Antivir Res 117:27–38. https://doi.org/10.1016/j.antiviral.2015.02.003
Hurt AC, Besselaar TG, Daniels RS et al (2016) Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2014-2015. Antivir Res 132:178–185. https://doi.org/10.1016/j.antiviral.2016.06.001
Gubareva LV, Besselaar TG, Daniels RS et al (2017) Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2015-2016. Antivir Res 146:12–20. https://doi.org/10.1016/j.antiviral.2017.08.004
Lackenby A, Besselaar TG, Daniels RS et al (2018) Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors and status of novel antivirals, 2016-2017. Antivir Res 157:38–46. https://doi.org/10.1016/j.antiviral.2018.07.001
Takashita E, Daniels RS, Fujisaki S et al (2020) Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2017-2018. Antivir Res 175:104718. https://doi.org/10.1016/j.antiviral.2020.104718
World Health Organization (2012) Meetings of the WHO working group on surveillance of influenza antiviral susceptibility – Geneva, November 2011 and June 2012. Wkly Epidemiol Rec 87(39):369–374
Tisdale M (2009) Chapter 31: Influenza M2 ion-channel and neuraminidase inhibitors. In: Mayers DL (ed) Antimicrobial drug resistance. Humana Press, pp 421–447. https://doi.org/10.1007/978-1-59745-180-2_31
Gubareva LV, Robinson MJ, Bethell RC et al (1997) Catalytic and framework mutations in the neuraminidase active site of influenza viruses that are resistant to 4-guanidino-Neu5Ac2en. J Virol 71(5):3385–3390. https://doi.org/10.1128/JVI.71.5.3385-3390.1997
Jonges M, Liu WM, van der Vries E et al (2010) Influenza virus inactivation for studies of antigenicity and phenotypic neuraminidase inhibitor resistance profiling. J Clin Microbiol 48(3):928–940. https://doi.org/10.1128/JCM.02045-09
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Takashita, E. (2022). Assays for Determining the Sialidase Activity of Influenza Viruses and Monitoring Influenza Virus Susceptibility to Neuraminidase Inhibitors. In: Suzuki, Y. (eds) Glycovirology. Methods in Molecular Biology, vol 2556. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2635-1_19
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2635-1_19
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2634-4
Online ISBN: 978-1-0716-2635-1
eBook Packages: Springer Protocols