Abstract
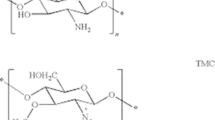
Drug delivery with appropriate dosage and interval at the target site by nanofabrication is the choice of research at present time. Use of polymers, viz., alginate, pectin, and chitosan, has been accepted by the European Pharmacopoeia as a newer process for structured drug delivery systems. Catechins, a predominant form of flavanols found naturally, have drawn particular consideration due to their relatively high antioxidant capacity in biological systems. The application of catechin is restricted because of its unstable nature in solution with reduced bioavailability in the body. Designing and nanofabrication not only decrease the repeated administration due to its sustained release properties to prevail over noncompliance but also facilitate the increase of the therapeutic value by reducing toxicity and escalating the bioavailability, stability, and target ability to the specific cell or organ. In this chapter, we have described nanofabrication of catechin-loaded alginate, pectin, and chitosan polymeric nanoparticles as a potent method to protect controlled release and to increase the action of bioactive compounds at the target cell or organ.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
De S, Robinson D (2003) Polymer relationships during preparation of chitosan–alginate and poly-L-lysine–alginate nanospheres. J Control Release 89:101–112
Motwani SK, Shruti C, Sushma T, Kanchan K, Farhan JA, Roop KK (2008) Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation, optimization and in vitro characterization. Eur J Pharm Biopharm 68:513–525
Ratnam V, Ankola DD, Bhardwaj V, Sahana DK, Ravi KMNV (2006) Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J Control Release 113:189–207
Tiyaboonchai W, Tungpradit W, Plianbangchang P (2007) Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int J Pharm 337:299–306
Brigger I, Dubernet C, Couvreur P (2002) Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev 54:631–651
Sun J, Tan H (2013) Alginate-based biomaterials for regenerative medicine applications. Materials (Basel) 6(4):285–1309. https://doi.org/10.3390/ma6041285
Yang CS (1999) Tea and health. Nutrition 15(11–12):946–949
Valcic S, Annette M, Neil EJ, Daniel CL, Barbara NT (1999) Antioxidant chemistry of green tea catechins. Identification of products of the reaction of (−)-epigallocatechingallate with peroxylradicals. Chem Res Toxicol 12:382–386
Jiang C, Jiao Y, Chen X, Li X, Yan W, Yu B, **ong Q (2013) Preliminary characterization and potential hepatoprotective effect of polysaccharides from Cipangopaludinachinensis. Food Chem Toxicol 59:18–25
Zhang L, Kosaraju SL (2007) Biopolymeric delivery system for controlled release of polyphenolic antioxidants. Eur Polym J 43(7):2956–2966
Ajazuddin SS (2010) Applications of novel drug delivery system for herbal formulations. Fitoterapia 81:680–689
Sang S, Lee MJ, Hou Z, Ho CT, Yang CS (2005) Stability of tea polyphenol (−) epigallocatechin-3-gallate and formation of dimmers and epimers under common experimental conditions. J Agric Food Chem 53:9478–9484
Tønnesen HH, Karlsen J (2002) Alginate in drug delivery systems. Drug Dev Ind Pharm 28(6):621–630
Pan JL, Bao ZM, Li JL, Zhang LG, Wu C, Yu YT (2005) Chitosan-based scaffolds for hepatocyte culture. ASBM6: AdvBiomat 6:91–94
Reis CP, Ribeiro AJ, Neufeld RJ, Veiga F (2007) Alginate microparticles as novel carrier for oral insulin delivery. Biotechnol Bioeng 96(5):977–989
Bonifácio BV, da Silva PB, dos Matheus Aparecido SR, Kamila M, Silveira N, Taís MB, Marlus C (2014) Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomedicine 9:1–15
Ansari SH, Farha I, Sameem M (2012) Influence of nanotechnology on herbal drugs: a review. J Adv Pharm Technol Res 3(3):140–146
Deljoo S, Rabiee N, Rabiee M (2019) Curcumin-hybrid nanoparticles in drug delivery system (review). Asian J Nanosci Mater 2:66–91
Peppas B, Blanchette JO (2004) Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev 56:1649–1659
Bhardwaj V, Hariharan S, Bala I, Lamprecht A, Kumar N, Panchagnula R, Kumar MNVR (2005) Pharmaceutical aspects of polymeric nanoparticles for oral delivery. J Biomed Nanotechnol 1:235–258
Tibbals HF (2011) Medical nanotechnology and nanomedicine. CRC Press, Boca Raton, Florida, pp 75–116
Rao JP, Geckeler KE (2011) Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci 36(7):887–913
Jana S, Gandhi A, Sen KK, Basu SK (2011) Natural polymers and their application in drug delivery and biomedical field. J Pharm Sci Technol 1:16–27
Tabata Y, Ikada Y (1989) Synthesis of gelatin microspheres containing interferon. Pharm Res 6:422–427
Onsøyen E (1996) Commercial applications of alginates. Carbohydr Eur 14:26–31
King AH (1983) In: Glicksman M (ed) In food hydrocolloids, vol 2. CRC Press, Boca Raton, pp 115–154
Soni MK, Kumar M, Namdeo KP (2010) Sodium alginate microspheres for extending drug release: formulation and in vitro evaluation. Int J Drug Deliv 2:64–68
Das RK, Naresh K, Utpal B (2010) Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine 6:153–160
Zahoor A, Rajesh P, Sadhna S, Khuller GK (2006) Alginate nanoparticles as antituberculosisdrug carriers: formulation development, pharmacokinetics and therapeutic potential. Indian J Chest Dis Allied Sci 48:171–176
Spizzirri UG, Ortensia IP, Francesca I, Giuseppe C, Francesco P, Manuela C, Nevio P (2010) Antioxidant–polysaccharide conjugates for food application by eco-friendly grafting procedure. Carbohydr Polym 79:333–340
Wicker L, Kim Y, Kim MJ, Thirkield B, Lin Z, Jung J (2014) Pectin as a bioactive polysaccharide–extracting tailored function from less. Food Hydrocoll 42:251–259
Muralikrishna G, Tharanathan RN (1994) Characterization of pectic polysaccharides from pulse husks. Food Chem 50(1):87–89
Da Silva JL, Rao MA (2007) Rheological behavior of food gels. In: Rheology of fluid and semisolid foods. Springer US, pp 339–401
Be Miller JN (1986) An introduction to pectin: structure and properties. In: Fishman ML, Jen JJ (eds) Chemistry andfunction of pectins, ACS symposium series 310. American Chemical Society, Washington, DC, pp 2–12
Löfgren C, Hermansson AM (2007) Synergistic rheological behaviour of mixed hm/lm pectin gels. Food Hydrocoll 21(3):480–486
Morris ER, Powell DA, Gidley MJ, Rees DA (1982) Conformations and interactions of pectins: I. polymorphism between gel and solid states of calcium polygalacturonate. J Mol Biol 155(4):507–516
Sandberg AS, Ahderinne R, Andersson H, Hallgren B, Hultén L (1983) The effect of citrus pectin on the absorption of nutrients in the small intestine. Hum Nutr Clin Nutr 37(3):171–183
Vandamme TF, Lenourry A, Charrueau C, Chaumeil JC (2002) The use of polysaccharides to target drugs to the colon. Carbohydr Polym 48(3):219–231
Liu L, Fishman ML, Hicks KB (2007) Pectin in controlled drug delivery–a review. Cellulose 14(1):15–24
Perera G, Barthelmes J, Bernkop-Schnürch A (2010) Novel pectin–4-aminothiophenole conjugate microparticles for colon-specific drug delivery. J Control Release 145(3):240–246
Ugurlu T, Turkoglu M, Gurer US, Akarsu BG (2007) Colonic delivery of compression coated nisin tablets using pectin/HPMC polymer mixture. Eur J Pharm Biopharm 67:202–210
Maestrelli F, Cirri M, Corti G, Mennini N, Mura P (2008) Development of enteric-coated calcium pectinate microspheres intended for colonic drug delivery. Eur J Pharm Biopharm 69:508–518
Katav T, Liu L, Traitel T, Goldbart R, Wolfson M, Kost J (2008) Modified pectin-based carrier for gene delivery: cellular barriers in gene delivery course. J Control Release 130:183–191
Thirawong N, Thongborisute J, Takeuchi H, Sriamornsak P (2008) Improved intestinal absorption of calcitonin by mucoadhesive delivery of novel pectin–liposome nanocomplexes. J Control Release 125(3):236–245
Sharma R, Ahuja M, Kaur H (2012) Thiolated pectin nanoparticles: preparation, characterization and ex vivo corneal permeation study. Carbohydr Polym 87(2):1606–1610
Verma AK, Chanchal A, Kumar A (2011) Potential of negatively charged pectin nanoparticles encapsulating paclitaxel: preparation and characterization. In: Nanoscience international conference on technology and societal implications, pp 1–8
Dutta RK, Sahu S (2012) Development of oxaliplatin encapsulated in magnetic nanocarriers of pectin as a potential targeted drug delivery for cancer therapy. Results Pharma Sci 2:38–45
Yu CY, Wang YM, Li NM, Liu GS, Yang S, Tang GT, Wei H (2014) In vitro and in vivo evaluation of pectin-based nanoparticles for hepatocellular carcinoma drug chemotherapy. Mol Pharm 11(2):638–644
Illum L (1998) Chitosan and its use as a pharmaceutical excipients. Pharm Res 15(9):1326–1331
Nagpal K, Singh SK, Mishra DN (2010) Chitosan nanoparticles: a promising system in novel drug delivery. Chem Pharm Bull 58(11):1423–1430
Li Q, Dunn ET, Grandmaison EW, Goosen MFA (1992) Applications and properties of chitosan. J Bioact Compat Polym 7:370–397
Janes KA, Calvo P, Alonso MJ (2001) Polysaccharide colloidal particles as delivery systems for macromolecules. Adv Drug Deliv Rev 47(1):83–97
Suzuki K, Mikami T, Okawa Y, Tokoro A, Suzuki S, Suzuki M (1986) Antitumor effect of hexa-N-acetylchitohexaose and chitohexaose. Carbohydr Res 151:403–408
Azuma K, Izumi R, Osaki T, Ifuku S, Morimoto M, Saimoto H, Minami S, Okamoto Y (2015) Chitin, chitosan, and its derivatives for wound healing: old and new materials. J Funct Biomater 6:104–142
Cheng K, Lim LY (2004) Insulin-loaded calcium pectinate nanoparticles: effects of pectin molecular weight and formulation in drug development. Ind Pharm 30(4):359–367
Zou Y, Yang Y, Li J, Li W, Wu Q (2006) Prevention of hepatic injury by a traditional Chinese formulation, BJ-JN in mice treated with bacille-calmette-guerin and lipopolysaccharide. J Ethnopharmacol 107:442–448
Altiok D, Altiok E, Tihminlioglu F (2010) J Mater Sci Mater Med 21:2227–2236
Davies NM, Farr SJ, Hadgraft J, Kellaway IW (1992) Evaluation of mucoadhesive polymers in ocular drug delivery. II-Polymer-coated vesicles. Pharm Res 9(9):1137–1144
Patel DP, Singh S (2015) Chitosan: a multifacet polymer. Int J Curr Pharm Res 7(2):21–28
Kotzé AF, Thanou MM, Luebetaen HL, De Boer AG, Verhoef JC, Junginger HE (1999) Enhancement of paracellular drug transport with highly quaternized N-trimethyl chitosan chloride in neutral environments: in vitro evaluation in intestinal epithelial cells. J Pharm Sci 88:253–257
Wu JL, Chao QW, Ren XZ, Si XC (2014) Multi-drug delivery system based on alginate/calcium carbonatehybrid nanoparticles for combination chemotherapy. Colloid Surf B Biointerfaces 123:498–505
Yu CY, Cao H, Zhang XC, Zhou FZ, Cheng SX, Zhang XZ, Zhuo RX (2009) Hybrid nanospheres and vesicles based on pectin as drug carriers. Langmuir 25(19):11720–11726
Calvo P, Remunan-Lopez C, Vilas-Jato JL, Alonso MJ (1997) Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carrier for protein and vaccines. Pharm Res 14(10):1431–1436
Liang HF, Yang TF, Huang CT, Chen MC, Sung HW (2005) Preparation of nanoparticles composed of poly(gamma-glutamic acid)-poly(lactide) block copolymers and evaluation of their uptake by HepG2 cells. J Control Release 105:213–225
Dudhani AR, Kosaraju SL (2010) Bioadhesive chitosan nanoparticles: preparation and characterization. Carbohydr Polym 81:243
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem 239(1):70–76
Basnet P, Matsumoo T, Neidlein R (1997) Potent free radical scavenging activity of propolis isolated from Brazilian Propolis. Naturfors 52(11–12):828–833
Ebrahimzadeh MA, Nabavi SM, Nabavi SF (2009) Correlation between the in vitro iron chelating activity and poly phenol and flavonoid contents of some medicinal plants. Pak J Biol Sci 12(12):934–938
Sayah MY, Chabir R, Benyahia H, RodiKandri Y, OuazzaniChahdi F, Touzani H, Errachidi F (2016) Yield, esterification degree and molecular weight evaluation of pectins isolated from orange and grapefruit peels under different conditions. PLoS One 11(9):e0161751. https://doi.org/10.1371/journal.pone.0161751
Sowjanya B, Prasanna RI, Devi KJ, Narayana S, Chetty CM, Purushothaman M, Gnanaprakash K (2010) Preparation and characterization of cefadroxil loaded alginate microbeads. Int J Res Pharm Sci 1(4):386–390
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Mondal, D.B., Velayudhan, J.M., Lekshman, A., Mandal, R.S.K., Raja, R., Kumar, N. (2022). Nanofabrication of Catechin-Loaded Alginate, Pectin, and Chitosan Polymeric Nanoparticles. In: Kumar, N., Kumar, V., Shrivastava, S., Gangwar, A.K., Saxena, S. (eds) Tissue Scaffolds. Springer Protocols Handbooks. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2425-8_31
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2425-8_31
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2424-1
Online ISBN: 978-1-0716-2425-8
eBook Packages: Springer Protocols