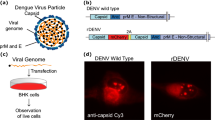
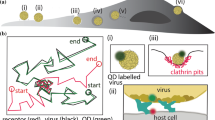
Abstract
It has become increasingly evident that unveiling the mechanisms of virus entry, assembly, and virion release is fundamental for identifying means for preventing viral spread and controlling viral disease. Due to virus mobility and structural and/or functional heterogeneity among viral particles, high spatiotemporal resolution single-virus/single-particle techniques are required to capture the behavior of viral particles inside infected cells.
In this chapter, we present fluorescence imaging analysis methods for studying the mobility of fluorescently labeled dengue virus (DENV) proteins in live infected cells. Some of the most recent Fluorescence Fluctuation Spectroscopy (FFS) methods will be presented and, in particular, the pair Correlation Functions (pCF) approach will be discussed. The pCF method does not require individual molecule isolation, as in a particle-tracking experiment, to capture single viral protein behavior. In this regard, image acquisition is followed by the spatiotemporal cross-correlation function at increasing time delays, yielding a quantitative view of single-particle mobility in intact live infected cells.
We provide a general overview and a practical guidance for the implementation of advanced FFS techniques, and the pair Correlation Functions analysis, as quantitative tools to reveal insights into previously unreported DENV mechanisms. We expect this protocol report will serve as an incentive for further applying correlation imaging studies in virology research.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Klumpp K, Crépin T (2014) Capsid proteins of enveloped viruses as antiviral drug targets. Curr Opin Virol 5:63–71
Oliveira ERA, Mohana-Borges R, de Alencastro RB et al (2016) The flavivirus capsid protein: structure, function, and perspectives towards drug design. Virus Res 227:115–123
Byk LA, Gamarnik AV (2016) Properties and functions of the dengue virus capsid protein. Annu Rev Virol 3(1):263–281
Samsa MM, Mondotte JA, Iglesias NG et al (2009) Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog 5(10):e1000632
Byk LA, Iglesias NG, De Maio FA et al (2016) Dengue virus genome uncoating requires ubiquitination. mBio 7(3):e00804–e00816
Chong MK, Chua AJ, Tan TT et al (2014) Microscopy techniques in flavivirus research. Micron 59:33–43
Zheng Y, Kielian M (2013) Imaging of the alphavirus capsid protein during virus replication. J Virol 87(17):9579–9589
Domingo E, de Ávila AI, Gallego I et al (2019) Viral fitness: history and relevance for viral pathogenesis and antiviral interventions. Pathog Dis 77(2):ftz021
Gabriel M, Moya-Díaz J, Gallo LI et al (2018) Single particle tracking of internalized metallic nanoparticles reveals heterogeneous directed motion after clathrin dependent endocytosis in mouse chromaffin cells. Methods Appl Fluoresc 6(1):014003
Coppola S, Estrada LC, Digman MA et al (2012) Intracellular trafficking of cationic liposome–DNA complexes in living cells. Soft Matter 8:7919–7927
Liu SL, Wang ZG, **e HY et al (2020) Single-virus tracking: from imaging methodologies to Virological applications. Chem Rev 120(3):1936–1979
Adu-Gyamfi E, Digman MA, Gratton E et al (2012) Single-particle tracking demonstrates that actin coordinates the movement of the Ebola virus matrix protein. Biophys J 103(9):L41–L43
Brandenburg B, Zhuang X (2007) Virus trafficking – learning from single-virus tracking. Nat Rev Microbiol 5(3):197–208
Kukura P, Ewers H, Müller C et al (2009) High-speed nanoscopic tracking of the position and orientation of a single virus. Nat Methods 6(12):923
Digman MA, Gratton E (2009) Imaging barriers to diffusion by pair correlation functions. Biophys J 97:665–673
Cardarelli F, Gratton E (2010) In vivo imaging of single-molecule trans-location through nuclear pore complexes by pair correlation functions. PLoS One 5:E10475
Hinde E, Cardarelli F, Digman MA et al (2010) In vivo pair correlation analysis of EGFP intranuclear diffusion reveals DNA-dependent molecular flow. PNAS 107(38):16560–16565
Malacrida L, Hedde PN, Ranjit S et al (2018) Visualization of barriers and obstacles to molecular diffusion in live cells by spatial pair-cross-correlation in two dimensions. Biomed Opt Express 9:691–697
Hedde PN, Staaf E, Singh SB et al (2019) Pair correlation analysis maps the dynamic two-dimensional Organization of Natural Killer Cell Receptors at the Synapse. ACS Nano 13(12):14274–14282
Gabriel M, Costa Navarro GS, de Borba L et al (2020) Dengue virus capsid protein dynamics reveals spatially heterogeneous motion in live-infected-cells. Sci Rep 10:8751
Hinde E, Cardarelli F, Digman MA et al (2012) Changes in chromatin compaction during the cell cycle revealed by micrometer-scale measurement of molecular flow in the nucleus. Biophys J 102:691–697
Ries J, Chiantia S, Schwille P (2006) Accurate determination of membrane dynamics with line-scan FCS. Biophys J 96(5):1999–2008
Ries J, Schwille P (2006) Studying slow membrane dynamics with continuous wave scanning fluorescence correlation spectroscopy. Biophys J 91(5):1915–1924
Digman MA, Gratton E (2011) Lessons in fluctuation correlation spectroscopy. Annu Rev Phys Chem 62:645–668
Gupta A, Sankaran J, Wohland T (2018) Fluorescence correlation spectroscopy: the technique and its applications in soft matter. Phys Sci Rev 4(4):20170104
Macháň R, Wohland T (2014) Recent applications of fluorescence correlation spectroscopy in live systems. Edited by Elias M. Puchner, Bo Huang, Hermann E. Gaub and Wilhelm Just. FEBS Lett 588:3571–3584
Elson E (2011) FCS past present and future. Biophys J 101:2855–2870
Bacia K, Schwille P (2007) Fluorescence correlation spectroscopy. In: Lipid rafts. Methods in molecular biology. Humana Press, Totowa, New Jersey, p 398. https://doi.org/10.1007/978-1-59745-513-8_7
Schwille P, Haustein E (2002) Fluorescence Correlation Spectroscopy. A tutorial for the Biophysics Textbook Online (BTOL). Biophysical Society, Rockville, MD
Schwille P (2001) Fluorescence correlation spectroscopy and its potential for intracellular applications. Cell Biochem Biophys 34(3):383–408
Hess ST, Webb WW (2002) Focal volume optics and experimental artifacts in confocal fluorescence correlation spectroscopy. Biophys J 83:2300–2317
Koppel D, Morgan F, Cowan A et al (1990) Scanning concentration correlation spectroscopy using the confocal laser microscope. Biophys J 66:502–507
Weissman M, Schindler H, Feher G (1976) Determination of molecular weights by fluctuation spectroscopy: application to DNA. Proc Natl Acad Sci U S A 73(8):2776–2780
Magde D, Elson EL, Webb WW (1972) Thermodynamic fluctuations in a reacting system measurement by fluorescence correlation spectroscopy. Phys Rev Lett 29:705–708
Magde D, Elson EL, Webb WW (1974) Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers 13:29–61
Berland KM, So PT, Gratton E (1995) Two-photon fluorescence correlation spectroscopy: method and application to the intracellular environment. Biophys J 68(2):694–701
Nagy A, Wu J, Berland KM (2005) Characterizing observation volumes and the role of excitation saturation in one-photon fluorescence fluctuation spectroscopy. J Biomed Opt 10(4):044015
Acknowledgments
This work was supported by University of Buenos Aires grant #PIDAE 2019-3444 and SINALA grant # 2019—L-AC4 to L.C.E., the National Institutes of Health (NIH-NIAID) R01.AI095175 to A.V.G and the National Institutes of Health (P41GM103540) to E.G. The authors gratefully acknowledge Alexis Luszczak for his help with simulations in Fig. 5.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix
Appendix
1.1 The (Very) Basics of Fluorescence Fluctuation Spectroscopy Techniques: History and New Challenges
The pCF approach described in this chapter belongs to a broader family of fluctuation-based methods known as Fluorescence Fluctuation Spectroscopy (FFS) and called Fluorescence Correlation Spectroscopy (FCS). FFS has grown in the last decades because of its multiple applications to study molecular dynamics in a wide variety of biological, chemical, and physical systems [24, 32, 33]. The original version of FFS , namely the point FFS approach, was introduced for the first time in 1972 by Magde, Elson, and Webb [34]. In their work, Magde and coworkers focused a laser beam on a solution containing a complex formed by both a drug and DNA. They demonstrated that, by analyzing the fluctuations of the collected fluorescence intensity originated by the variation in the emission quantum yield of the fluorophores when bound to DNA, it was possible to determine the concentration of each component. Later, in 1974, the same group described the capabilities of the method to measure the diffusion coefficient and kinetic constants of fluorescent molecules in solution [35]. These pioneering experiments triggered a variety of new techniques based on the intensity fluctuation principle. One of the main advantages of these approaches is that it is not required to externally perturb the system. Instead, the FFS uses the spontaneous fluctuations at equilibrium of the system under study, making them suitable for the study of intracellular dynamics in live organisms.
Several excellent reviews have been written describing the theory of FFS-based techniques and the reader is directed to these sources for an in-depth theoretical discussion [24, 28, 29]. Summarized below are the basic principles and underlying statistical analyses used to extract molecular dynamics values from fluorescence fluctuation measurements and correlation analysis. Briefly, FFS techniques have proved to be powerful tools to determine, with extreme sensitivity, molecular diffusion properties, local concentration, molecular interactions, and conformational changes down to the level of single molecules [24, 25, 27].
The simplest FCS approach consists of illuminating a sample with a focused laser beam in a fixed position of the sample and measuring the collected fluorescence as a function of time (Fig. 6). Intensity fluctuations can originate from molecules moving in and out of the observation volume of a confocal or two-photon microscope, or from intensity fluctuations due to photophysical processes such as blinking, changes in orientation, or conformational transitions. In any case, as originally described by [34, 35], molecular dynamics can be obtained by computing the time auto-correlation function (ACF) or G(τ) of the fluorescence intensity as:
Schematic representation of a single-point FCS experiment. (a) Molecules diffusing in and out of the observation volume. (b) Intensity traces over time. Molecular diffusion produces fluctuations on the detected fluorescence intensity. Aggregates (schematized as big red circles) diffuse slower than monomers (schematized as small green circles), so the duration of red fluctuations are longer in time. Increasing the molecular concentration (blue circles) the fluctuations have smaller amplitudes. (c) The temporal auto-correlation function (also called ACF) computed over different time delays, τ. This case is equivalent to the pCF at zero distance, pCF(0), because no scanning is performed. The amplitude is proportional to γ/<N> where <N> is the average number of molecules in the observation volume and γ a geometrical factor which depends on the 3D shape of the microscope observation volume
where F(t) is the fluorescence intensity collected at time t, and τ is the time lag. The brackets <> indicate sum over all measured time points. In the mathematical framework of pCF, the ACF is the analogous of the pCF(0), the pair correlation function computed at zero distance. In the case of intensity variations due to free pure diffusion, the average duration of the fluctuations is determined by the mean transit time of molecules to traverse the observation volume. The transit time (or correlation time τc) depends on the diffusion coefficient D and by the lateral waist size of the observation volume ω0 by τc = ω02/nD, where n is a geometrical factor equals to 4 for confocal or 8 for two-photon systems [24, 36]. The average amplitude of the fluctuations depends inversely on the average number of molecules in the observation volume [24].
The shape and size of the observation volume is given by the microscope settings, the microscope objective, the excitation wavelength, and other factors. It has been shown [29, 37] that its shape is well described by a 3D Gaussian function for a confocal microscope or a 2D Gaussian-Lorentzian function for a two-photon setup. However, many chemical, physical, and biological processes occur in large spatial scales and cannot be completely studied by observing a single “point” in the cell interior. A protein, for example, can move along the whole cytoplasm, bordering membranes or confining at specific regions.
To overcome this limitation, it is possible to sequentially perform point FCS experiments at different positions of the sample (i.e., in a scanned line) computing ACF curves along the pixels of the scanned lines. This approach is suitable for having quantitative information at different regions of the sample, i.e., the diffusion coefficient at different compartments of the cell or at different positions of the same compartment. In a commercial microscope, this experiment can be simply performed by periodically moving the excitation beam in a line of N pixels over the sample while molecules diffuse. Some of these molecules will be illuminated at many points as they follow a movement like the scanned line, while other molecules will follow different paths and then will be only illuminated during a few pixels over the line. Computing the ACF at subsequent points along a line has expanded the capabilities of the point FCS method to capture the dynamic in the cell interior. However, this approach does not allow us to relate what is happening between different regions, such as molecular translocation or nucleocytoplasmic shuttling of viral proteins. To overcome this limitation, Gratton and collaborators have introduced the concept of the pair Correlation Function (pCF) whereby correlating the fluorescence intensity fluctuations at a pair of specific points in a scanned line returns the time it takes a molecule to move (diffusing or not) between the two locations. As previously noted in this chapter, the pCF approach combines the advantages of FFS and SPT methodologies returning the time needed for each molecule to be found in each point in space. If a barrier to diffusion is present, a longer time will be needed for the same molecule to be found at a position across the barrier, as already demonstrated in the case of molecular transport across the NE [16]. Thus, FFS techniques and more specifically the pCF approach are extremely appropriate to study molecular shuttling across the NE and transport across other intracellular components [17, 21]. For further and deeper discussion on the discussed techniques, readers are referred to specialized literature [15, 17,18,19, 24].
Finally, we expect this work will help to stimulate the use of advanced FFS techniques, in particular the pCF, for dissecting molecular processes for viral infection, which is a fundamental step in identifying control measures.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Gabriel, M.V., Sallaberry, I., Costa Navarro, G.S., Gratton, E., Gamarnik, A.V., Estrada, L.C. (2022). Dengue Virus Capsid-Protein Dynamics in Live Infected Cells Studied by Pair Correlation Analysis. In: Mohana-Borges, R. (eds) Dengue Virus. Methods in Molecular Biology, vol 2409. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1879-0_8
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1879-0_8
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1878-3
Online ISBN: 978-1-0716-1879-0
eBook Packages: Springer Protocols