Abstract
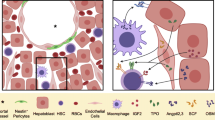
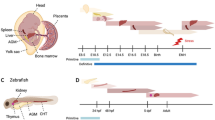
Hematopoietic stem cells (HSCs) are used in the clinic to provide life-saving therapies to patients with a variety of hematological malignancies and disorders. Yet, serious deficiencies in our understanding of how HSCs develop and self-renew continue to limit our ability to make this therapy safer and more broadly available to those who have no available donor. Finding ways to expand HSCs and develop alternate sources of HSCs is an urgent priority. In the embryo, a critical transition in development of the blood system requires that newly emergent HSCs from the aorta-gonad-mesonephros (AGM) region migrate to the fetal liver where they aggressively self-renew and expand to numbers sufficient to sustain the adult long term. This process of homing to the fetal liver is orchestrated by intrinsic regulators such as epigenetic modifications to the genome, expression of transcription factors, and adhesion molecule presentation, as well as sensing of extrinsic factors like chemokines, cytokines, and other molecules. Due to technical limitations in manipulating the fetal tissue microenvironment, mechanisms mediating the homing and expansion process remain incompletely understood. Importantly, HSC development is strictly dependent upon forces created by the flow of blood, and current experimental methods make the study of biophysical cues especially challenging. In the protocol presented herein, we address these limitations by designing a biomimetic ex vivo microfluidic model of the fetal liver that enables monitoring of HSC homing to and interaction with fetal liver niches under flow and matrix elasticity conditions typical during embryonic development. This model can be easily customized for the study of key microenvironmental factors and biophysical cues that support HSC homing and expansion.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Bailey C, Richardson LC, Allemani C et al (2018) Adult leukemia survival trends in the United States by subtype: a population-based registry study of 370,994 patients diagnosed during 1995-2009. Cancer 124:3856–3867
Tamplin OJ, Durand EM, Carr LA et al (2015) Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 160:241–252
Chou S, Lodish HF (2010) Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. Proc Natl Acad Sci U S A 107:7799–7804
Ema H, Nakauchi H (2000) Expansion of hematopoietic stem cells in the develo** liver of a mouse embryo. Blood 95:2284–2288
Ghiaur G, Ferkowicz MJ, Milsom MD et al (2008) Rac1 is essential for intraembryonic hematopoiesis and for the initial seeding of fetal liver with definitive hematopoietic progenitor cells. Blood 111:3313–3321
Kim I, Yilmaz ÖH, Morrison SJ (2005) CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood 106:903–905
Hirsch E, Iglesias A, Potocnik AJJ et al (1996) Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature 380:171–175
Manesia JK, Franch M, Tabas-Madrid D et al (2017) Distinct molecular signature of murine fetal liver and adult hematopoietic stem cells identify novel regulators of hematopoietic stem cell function. Stem Cells Dev 26:573–584
Teitell MA, Mikkola HKA (2006) Transcriptional activators, repressors, and epigenetic modifiers controlling hematopoietic stem cell development. Pediatr Res 59:33–39
Sommerkamp P, Renders S, Ladel L et al (2019) The long non-coding RNA Meg3 is dispensable for hematopoietic stem cells. Sci Rep 9:1–11
Mayani H (2016) The regulation of hematopoietic stem cell populations. F1000 Res 5:1524
Khan JA, Mendelson A, Kunisaki Y et al (2016) Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 351:176–180
Joseph C, Quach JM, Walkley CR et al (2013) Deciphering hematopoietic stem cells in their niches: a critical appraisal of genetic models, lineage tracing, and imaging strategies. Cell Stem Cell 13:520–533
Zhang P, Zhang C, Li J et al (2019) The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res Ther 10:327
Adamo L, Naveiras O, Wenzel PL et al (2009) Biomechanical forces promote embryonic haematopoiesis. Nature 459:1131–1135
North TE, Goessling W, Peeters M et al (2009) Hematopoietic stem cell development is dependent on blood flow. Cell 137:736–748
Diaz MF, Li N, Lee HJ et al (2015) Biomechanical forces promote blood development through prostaglandin E2 and the cAMP-PKA signaling axis. J Exp Med 212:665–680
Tsai HA, Shen CN, Chang YC (2012) Use of surface properties to control the growth and differentiation of mouse fetal liver stem/progenitor cell colonies. Biomacromolecules 13:3483–3493
Ulrich TA, De Juan Pardo EM, Kumar S (2009) The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res 69:4167–4174
Saha K, Keung A, Irwin E et al (2008) Substrate modulus directs neural stem cell behavior. Biophy J 95:4426–4438
Frye M, Taddei A, Dierkes C et al (2018) Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program. Nat Commun 9:1–16
Nagasaka A, Shinoda T, Kawaue T et al (2016) Differences in the mechanical properties of the develo** cerebral cortical proliferative zone between mice and ferrets at both the tissue and single-cell levels. Front Cell Dev Biol 4:1–13
Chevalier NR, Gazquez E, Bidault L et al (2016) How tissue mechanical properties affect enteric neural crest cell migration. Sci Rep 6:1–17
Iwashita M, Kataoka N, Toida K et al (2014) Systematic profiling of spatiotemporal tissue and cellular stiffness in the develo** brain. Development 141:3793–3798
Acknowledgments
This work was supported by the American Society of Hematology Scholar Award and a grant from the National Institutes of Health (R01DK111599) to P.L.W.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Science+Business Media New York
About this protocol
Cite this protocol
Mohammadalipour, A., Diaz, M.F., Pareek, S., Wenzel, P.L. (2020). Ex Vivo Modeling of Hematopoietic Stem Cell Homing to the Fetal Liver. In: Turksen, K. (eds) Stem Cell Renewal and Cell-Cell Communication. Methods in Molecular Biology, vol 2346. Humana, New York, NY. https://doi.org/10.1007/7651_2020_293
Download citation
DOI: https://doi.org/10.1007/7651_2020_293
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1569-0
Online ISBN: 978-1-0716-1570-6
eBook Packages: Springer Protocols