Abstract
Infections caused by respiratory viruses can have different clinical symptoms, while specific set of symptoms can be induced by different viruses. Despite usually mild course of disease, some viruses causing certain disease entity can result in serious complications. Therefore, quick and appropriate diagnostic is crucial for administering proper treatment. In the epidemic season 2013/2014, 2,497 specimens were tested. Infections caused by influenza viruses were confirmed in 9.8 %, while infections caused by influenza-like viruses (ILI) in 13.2 %. The co-domination of A/H1N1/pdm09 (29.4 %) with A/H3N2/ (30.6 %) was observed among circulating subtypes of influenza virus type A. Analysis of positive specimens categorized into 7 age groups indicated that most of morbidity to influenza was noted in the age intervals: 26–44 (22.9 %) and 45–64 years old (21.6 %). Considering infections caused by influenza-like viruses, the highest amount of positive cases was registered in the age group 0–4 years old (92.7 %) with the highest ratio of RSV (87.9 %) and PIV-3 (10.5 %). Judging by the epidemiological and virological indicators, the 2013/14 influenza season was mild and only low virus activity was reported in Poland as well as in most European countries. Still, 9,000 hospitalizations and 17 deaths were registered in Poland during this epidemic season.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Respiratory tract infections consist one of the major health problem of society, because they lead to high rates of hospitalizations, morbidity, and mortality. Essential feature of respiratory viruses is their easiness of transmission, especially in places with great density of people (schools, governments, mall centers, and means of public transport) (Brydak 2007). Elder people, children up to 24 months and persons from high risk groups are particularly exposed to respiratory viruses’ infections. The most frequently circulating respiratory viruses are: influenza virus type A and B, respiratory syncytial virus A (RSV-A) and B (RSV-B), parainfluenza viruses 1–3 (PIV-1,-2,-3), human coronavirus (hCoV), adenovirus (ADV), human rhinovirus (hRV), and human metapneumovirus (hMPV). Infections caused by respiratory viruses can have different clinical symptoms, while specific set of symptoms can be induced by different viruses. Despite usually mild course of disease, some viruses causing certain disease entity can result in serious complications. Furthermore, not only viruses can cause particular illness, but also bacteria. Therefore, quick and appropriate diagnostic is crucial for administering proper treatment (Stefańska et al. 2013). What is more, organism attacked by one pathogen has reduced immunity and becomes vulnerable to the subsequent ones. In this way numerous co-infections occur, which can result in death (Brydak 2008, 2011).
2 Methods
2.1 Patient Population and Specimen Collection
In the epidemic season 2013/2014, 2,471 clinical specimens were tested in Poland. Additionally, National Influenza Center (NIC), Department of Influenza Research at National Institute of Public Health – National Institute of Hygiene as a Reference Laboratory in Poland – tested selected specimens from Voivodeship Sanitary Epidemiological Stations (VSES) to verify the results obtained in these labs. Mainly, materials for testing consisted from nasal and throat swabs, while in NIC also bronchus-associated lymphoid fluid (BALF) was tested. The age of the patients ranged from 1 day to 87 years of age. The patients were categorized into 7 age groups: 0–4, 5–9, 10–14, 15–25, 26–44, 45–64, and >65 years of age.
2.2 Extraction of Viral RNA
The viral RNA was extracted using a Maxwell 16 Viral Total Nucleic Acid Purification Kit (Promega Corporation, Madison, WI) from 200 μl of clinical samples in viral transport medium (PBS), in accordance with the manufacturer’s instructions for low elution volume (LEV) cartridges. The RNA was eluted with 50 μl of RNase-free water.
2.3 Real-Time Polymerase Chain Reaction (RT-PCR)
Because RNA virus samples were typed in Voivodeship Sanitary Epidemiological Stations, only subty** of influenza viruses was achieved. The detection of subtypes was performed by RT-PCR. One-step RT-PCR analysis was performed using Roche Light Cycler 2.0 System. RT-PCR reactions were performed in capillary tubes in 20 μl volumes with 0.5 μM primers and 0.2 μM probe for each reaction. According to WHO recommendations. The primers GRswH1-349Fw, GRswH1-601Rv, GRsw-N1-975Fw, GRswN1-1084Rv, H3h-319Fw, H3h-377Rv, N2h-1150bFw, N2h-1344Rv, and probes GRswH1-538, GRswN1-10465b, H3h-358, N2h1290 were used (WHO 2014). The reaction mixture, containing reaction buffer, MgSO4 buffer, BSA, RNase-free H2O, and SuperScript® III/Platinum® Taq Mix, was incubated with 5 μl RNA sample per capillary tube. RNA from vaccine viruses A/California/7/2009(H1N1)pdm09 and A/Victoria/361/2011(H3N2) were introduced as the positive control sample and RNase-free H2O was utilized as the negative control sample. Before DNA amplification cycles were begun, the RNA templates were reverse transcribed to produce the corresponding cDNA templates during reverse transcription procedure: 45 °C for 15 min. DNA templates were then subjected to an initialization step (1 cycle at 95 °C for 3 min), followed by 50 cycles of amplification: denaturation at 95 °C for 10 s, annealing at 55 °C for 10 s and elongation at 72 °C for 20 s.
2.4 Conventional Multiplex RT-PCR
Thirty six specimens from patients, stored at −80 °C, were tested by RT-PCR using RV12 ACE Detection Kit (Seegene, Seoul, South Korea) that enables detection of following respiratory viruses: Influenza A virus, influenza B virus, human respiratory syncytial virus A, human respiratory syncytial virus B, human adenovirus, human metapneumovirus, human coronavirus 229E/NL63, human coronavirus OC43, human parainfluenza virus 1, human parainfluenza virus 2, human parainfluenza virus 3, and human rhinovirus A/B. Random hexamer –primed cDNA synthesis products were generated using the first strand cDNA synthesis kit (Fermentas, York, UK), according to the manufacturer’s instructions, and stored at −20 °C until use.
Each cDNA preparation was subjected to the RV12 PCR procedure according to the manufacturer’s instructions (Seegene, Seoul, South Korea). Afterward, amplicons were detected by gel electrophoresis.
3 Results
In the epidemic season 2013/2014, 2,497 specimens were tested. 23.0 % were positive for influenza and influenza-like viruses. Infections caused by influenza viruses were confirmed in 9.8 %, while infections caused by influenza-like viruses in 13.2 % (Table 1).
The majority of influenza-like cases – 57.4 % was caused by RSV (83.9 %). Infections of upper respiratory tracts were also caused by viruses such as: PIV-3 (11.2 %), rhinovirus (1.8 %), adenovirus (1.2 %), human coronavirus (0.6 %), metapneumovirus (0.6 %), PIV-1 (0.3 %), and PIV-2 (0.3 %). Taking into account infections caused only by influenza viruses, they amounted to 42.6 % of all laboratory confirmed cases. The predominant type of influenza virus was type A which made up 98.8 % of all influenza cases. In the epidemic season 2013/2014, co-domination of A/H1N1/pdm09 (29.4 %) with A/H3N2/ (30.6 %) was observed among circulating subtypes of influenza virus type A. Influenza virus type B comprised 1.2 % of influenza virus confirmations.
Analysis of positive specimens categorized into 7 age groups indicated, that most of morbidity to influenza was noted in age groups: 26–44 (22.9 %) and 45–64 years of age (21.6 %), whereas the influenza was the rarest in the age group 10–14 years of age (5.3 %) (Table 2). If it goes about influenza laboratory confirmed cases in high risk groups, 14.7 % of specimens were positive in >65 years age group and 17.6 % in 0–4 years age group. In all seven age groups, a co-domination between two subtypes of influenza virus type A was present, i.e. A/H1N1/pdm09 and A/H3N2/. Considering infections caused by influenza-like viruses, the biggest amount of positive cases was registered in the age group 0–4 years of age (92.7 %), with the highest ratio of RS virus (87.9 %) and PIV-3 (10.5 %). The lowest rate of infections caused by influenza-like viruses was observed in age groups: >65 (0.3 %) and 10–14 years of age (0.3 %).
Verification of results received by VSESs demonstrated complete consistency with the results obtained NIC (Table 3). In NIC, infections caused by influenza viruses comprised 50 % of laboratory confirmed cases and the other 50 % were those caused by influenza-like viruses. In the event of influenza viruses, the predominant subtype was A/H3N2/ (50 %), while A/H1N1/pdm09 made up 18.8 % of confirmations.
Taking into consideration only influenza-like viruses, it was noted that RSV (31.3 %) and Rhinovirus (37.5 %) were dominating in this group. Moreover, eight co-infections were registered in the framework of conducted research. Five of them were between influenza virus and influenza-like viruses (A + ADV, 2 A + RSV +hRV, A/H3N2/ + RSV B, A/H3N2/ + hCoV), whereas three co-infections were detected only among influenza-like viruses (RSV A + hRV, RSV B + PIV-3, ADV + PIV-1-3 + hCoV). All co-infections detected in epidemic season 2013/2014 in Poland are presented in Table 4.
4 Discussion
In the epidemic season 2013/2014 in Poland, 2,497 specimens were tested, 23.0 % of which consisted positive specimens, which was, for comparison, about one third of the 6,949 tests performed in the preceding season. In order to get detailed virological analysis, laboratory data were categorized into 7 age groups: 0–4, 5–9, 10–14, 15–25, 26–54, 45–64, and >65 years old. Influenza viruses made up 42.6 %, while influenza-like viruses 57.4 % of all positively confirmed cases. Non-subtyped influenza virus type A was present in 39.3 % specimens and quite close percentages concerned subtypes A/H3N2/ – 31.0 % and A/H1N1/pdm09 – 29.8 %. In the season, three types of co-infections were registered: co-infections within influenza viruses, influenza viruses with influenza-like viruses, and co-infections within influenza-like viruses. Detailed information is presented in Table 1. Co-infection combinations encountered, especially the one combining five influenza-like viruses, were rather unprecedented and registered for the first time in Poland.
In the epidemic season 2012/2013, the dominant type of influenza virus was type A (86.8 %), while influenza virus type B was confirmed in 9.8 %. A significantly lower number of circulating influenza virus type B was noted in Poland compared with many a previous epidemic season. The same observations were made in Europe and worldwide. Explicitly fewer cases of infections caused by influenza virus type B were registered in the epidemic season 2013/2014, than in previous seasons – only 1.2 % of confirmations. Concerning influenza virus type A, 66.7 % of confirmations were A/H1N1/pdm09, whereas A/H3N2/ amounted merely to 11.7 %. Non-subtyped influenza viruses type A constituted 21.6 % of influenza type A confirmations. Among influenza-like viruses, RSV was the prevalent one (87.0 %).
In every epidemic season, infections caused by influenza virus and influenza-like viruses are registered. In the epidemic season 2013/2014, the highest number of influenza confirmations was registered in the age group 26–44 years old (22.8 %), followed by 45–64 years old (21.6 %), and the lowest percentage was in 10–14 years old (5.3 %). In case of children aged 0–4 years old, an alarming issue is that the percentage of vaccinated children has been remaining at a very low level, namely 0.6–0.9 %, throughout many epidemic seasons. Despite seasonal recommendations of Advisory Committee on Immunization Practices and many other international scientific societies, the percentage of vaccinated children in Poland remains disappointingly low (Epimeld 2014; Epperson et al. 2014; Grohskopf et al. 2014).
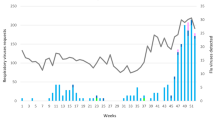
Judging by the epidemiological and virological indicators, the epidemic season 2013/14 was mild and only low virus activity was reported in Poland as well as in most European countries. The occurrence of influenza has been delayed in Europe in the epidemic season 2013/2014, with a different timing across European countries. In the first four affected countries (Bulgaria, Greece, Portugal, and Spain), A/H1N1/pdm09 virus dominated. The peak of incidence to influenza and influenza-like infections in Europe occurred in the third week of 2014 (13–19. 01. 2014), whereas in Poland it was in the eleventh week of 2014 (10–16. 03. 2014) (Fig. 1). At the beginning of the season, A/H1N1/pdm09 virus dominated, while in the last weeks of the season, A/H3N2/ virus gained prevalence.
According to the NIPH-NIH information, 9,000 hospitalizations and 17 deaths were registered in Poland in 2013/2014. However, data regarding deaths are definitely underestimated, because only 3.8 % of population was vaccinated against influenza. In Europe, 430 deaths of influenza were noted with the highest number in Spain (ECDC 2014).
References
Brydak LB (2007) Diagnostics and prophylaxis of respiratory tract infections – the latest findings. Przewodnik Lekarza 2(94):147–153
Brydak LB (2008) Influenza, pandemic flu, myth or real threat? Rythm, Warszawa (in Polish)
Brydak LB (2011) Infections caused by respiratory viruses and the possibilities of controlling them. Pol Merkur Lekarski 30:179–355
ECDC surveillance report, Influenza in Europe – season 2013–2014. Available from: http://www.ecdc.europa.eu/en/publications/Publications/Influenza-2013-14-season-report.pdf. Accessed on 3 Sept 2014
Epimeld (2014) Influenza and influenza-like illness. Available from: http://www.pzh.gov.pl/oldpage/epimeld/grypa/index.htm. Accessed on 25 Aug 2014
Epperson S, Blanton L, Kniss K, Mustaquim D, Steffens C, Wallis T, Dhara R, Leon M, Perez A, Chaves SS, Elal AA, Gubareva L, Xu X, Villanueva J, Bresee J, Cox N, Finelli L, Brammer L (2014) Influenza activity – United States, 2013–14 season and composition of the 2014–15 influenza vaccines. Morb Mortal Wkly Rep 63(22):483–490
Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, Karron RA, Walter EB (2014) Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) – United States, 2014–15 influenza season. Morb Mortal Wkly Rep 63(32):691–697
Stefańska I, Romanowska M, Donevski S, Gawryluk D, Brydak LB (2013) Co-infections with influenza and other respiratory viruses. Adv Exp Med Biol 756:291–301
WHO (2014) WHO information for molecular diagnosis of influenza virus – update. Available from: http://www.who.int/influenza/gisrs_laboratory/molecular_diagnosis_influenza_virus_humans_update_201403.pdf?ua=1
Acknowledgements
We would like to acknowledge physicians and employees of VSESs participating in SENTINEL program for their input into the influenza surveillance in Poland. The project was partially supported by the Ministry of Science and Education (Grant No 2011/01/B/NZ7/06188) and NIPH-NIH funds (8/EM/6K/2013, 8/EM/11K/2014).
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bednarska, K., Hallmann-Szelińska, E., Kondratiuk, K., Brydak, L.B. (2015). Evaluation of the Activity of Influenza and Influenza-Like Viruses in the Epidemic Season 2013/2014. In: Pokorski, M. (eds) Pulmonary Infection. Advances in Experimental Medicine and Biology(), vol 857. Springer, Cham. https://doi.org/10.1007/5584_2015_116
Download citation
DOI: https://doi.org/10.1007/5584_2015_116
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17457-0
Online ISBN: 978-3-319-17458-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)