Abstract
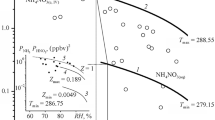
Estimates for the acidity of aerosol particles in the rural atmosphere over Wingene (Belgium) are presented. The results of processing monitoring data using data of the control of meteorological conditions, gas impurities, ion composition, and mass concentration of aerosol particles, as well as the use of thermodynamic methods showed that the level of acidity of aerosol particles in the atmosphere is unexpectedly high (\(\mathrm{pH}\approx 3.5{-}4.3\)) despite their sampling in the background area. It is found that the variability of particle acidity is mainly determined by fluctuations in relative humidity and the content of gas-phase ammonia in the air. In this case, the lower the relative humidity is, the higher the acidity of particles is, which reflects the variability of moisture content in particles and the solubility of ammonium nitrate.

Similar content being viewed by others
REFERENCES
A. N. Yermakov, A. E. Aloyan, and V. O. Arutyunyan, “Seasonal Variability of the Ion Composition, Phase State, and Mass Concentration of Aerosol in the Rural and Urban Atmosphere over Belgium (2001–2003),” Meteorol. Gidrol., No. 3 (2020) [Russ. Meteorol. Hydrol., No. 3, 45 (2020)].
A. N. Yermakov, A. E. Aloyan, T. V. Khodzer, L. P. Golobokova, and V. O. Arutyunyan, “On the Influence of Atmospheric Chemical Reactions on the Ion Composition of Aerosol Particles in the Baikal Region,” Izv. Akad. Nauk, Fiz. Atmos. Okeana, No. 2, 43 (2007) [Izv., Atmos. Oceanic Phys., No. 2, 43 (2007)].
A. N. Yermakov, L. P. Golobokova, O. G. Netsvetaeva, A. E. Aloyan, V. O. Arutyunyan, and T. V. Khodzher, “On the Nature of Aerosol Particles in the Atmosphere of Irkutsk,” Izv. Akad. Nauk, Fiz. Atmos. Okeana, No. 2, 54 (2018) [Izv., Atmos. Oceanic Phys., No. 2, 54 (2018)].
R. A. Robinson and R. H. Stokes, Electrolyte Solutions (Inostrannaya Literatura, Moscow, 1963) [in Russian].
B. V. Romanovskii, Catalysis Fundamentals (Binom: Laboratoriya Znanii, Moscow, 2017) [in Russian].
H. Akimoto, Atmospheric Reaction Chemistry (Springer Japan, 2016).
G. P. Brasseur, J. J. Orlando, and G. S. Tyndall, Atmospheric Chemistry and Global Change (Oxford University Press, New York, 1999).
CCSP 2009: Atmospheric Aerosol Properties and Climate Impacts, A Report by the U.S. Climate Change Science Program and the Subcommittee on Global Change Research, Ed. by M. Chin, R. A. Kahn, and S. E. Schwartz (NASA, Washington, DC, 2009).
S. L. Clegg, P. Brimblecombe, and A. S. Wexler, “A Thermodynamic Model of the System H\(^{ + }\)–NH\(_4^+\)–SO\(_4^{2-}\)–NO\(_3^-\)–H\(_2\)O at Tropospheric Temperatures,” J. Phys. Chem. A, No. 12, 102 (1998).
S. Gao, N. L. Ng, M. Keywood, V. Varutbangkul, R. Bahreini, A. Nenes, J. He, K. Yoo, J. Beauchamp, R. Hodyss, R. Flagan, and J. Seinfeld, “Particle Phase Acidity and Oligomer Formation in Secondary Organic Aerosol,” Environ. Sci. Technol., No. 24, 38 (2004).
R. V. Grieken, Optimization and Environmental Application of TW-EPMA for Single Particle Analysis (Antwerpen University, 2005).
http://www.aim.env.uea.ac.uk/aim/aim.php.
M. Z. Jacobsen, “Development and Application of a New Air Pollution Modeling System. II. Aerosol Module Structure and Design,” Atmos. Environ., 31 (1997).
M. Jang, N. M. Czoschke, S. Lee, and R. M. Kamens, “Heterogeneous Atmospheric Aerosol Production by Acid Catalyzed Particle-phase Reactions,” Science, 298 (2002).
M. Kanakidou, J. H. Seinfeld, S. N. Pandis, I. Barnes, F. J. Dentener, M. C. Facchini, R. Van Dingenen, B. Ervens, A. Nenes, C. J. Nielsen, E. Swietlicki, J. P. Putaud, Y. Balkanski, S. Fuzzi, J. Horth, G. K. Moortgat, R. Winterhalter, C. E. L. Myhre, K. Tsigaridis, E. Vignati, E. G. Stephanou, and J. Wilson, “Organic Aerosol and Global Climate Modeling: A Review,” Atmos. Chem. Phys., No. 4, 5 (2005).
J. H. Kroll, A. W. H. Chan, N. L. Ng, R. C. Flagan, and J. H. Seinfeld, “Reactions of Semivolatile Organics and Their Effects on Secondary Organic Aerosol Formation,” Environ. Sci. Technol., No. 10, 41 (2007).
Z. F. Lu, J. M. Hao, H. Takekawa, L. H. Hu, and J. H. Li, “Effect of High Concentrations of Inorganic Seed Aerosols on Secondary Organic Aerosol Formation in the m-xylene/NO\(_{x}\) Photooxidation System,” Atmos. Environ., No. 4, 43 (2009).
A. Nenes, S. N. Pandis, and C. Plinis, “ISORROPIA: A New Thermodynamic Equilibrium Model for Multiphase Multicomponent Inorganic Aerosols,” Aquatic Geochem., 4 (1998).
C. Pilinis and J. H. Seinfeld, “Continued Development of a General Equilibrium Model for Inorganic Multicomponent Atmospheric Aerosols,” Atmos. Environ., 21 (1987).
J. H. Seinfeld and S. N. Pandis, Atmospheric Chemistry and Physics. From Air Pollution to Climate Change (Wiley-Interscience, New York, 1997).
J. E. Shilling, Q. Chen, S. M. King, T. Rosenoern, J. Kroll, D. Worsnop, P. DeCarlo, A. Aiken, D. Sueper, J. Jimenez, and S. T. Martin, “Loading-dependent Elemental Composition of \(\alpha\)-pinene SOA Particles,” Atmos. Chem. Phys., 9 (2009).
A. W. Stelson, M. E. Basset, and J. H. Seinfeld, “Thermodynamic Equilibrium Properties of Aqueous Solutions of Nitrate and Sulfate Ammonium,” in Chemistry of Particles, Fogs and Rain, Ed. by J. L. Duham (Butterworth-Heinemann, Newton, Mass., 1984).
Y. Zhang, B. Pun, K. Vijayaraghavan, S.-Y. Wu, C. Seigneur, S. Pandis, M. Jacobson, A. Nenes, and J. Seinfeld, “Development and Application of the Model of Aerosol Dynamics, Reaction, Ionization and Dissolution (MADRID),” J. Geophys. Res., 109 (2004).
R. Zhao, C. M. Kenseth, Y. Huang, N. Dalleska, X. M. Kuang, J. Chen, S. Paulson, and J. Seinfeld, “Rapid Aqueous-phase Hydrolysis of Ester Hydroperoxides Arising from Criegee Intermediates and Organic Acids,” J. Phys. Chem. A., No. 23, 122 (2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Meteorologiya i Gidrologiya, 2021, No. 11, pp. 56-63. https://doi.org/10.52002/0130-2906-2021-11-56-63.
About this article
Cite this article
Yermakov, A.N., Aloyan, A.E. & Arutyunyan, V.O. Acidity of Aerosol Particles in the Rural Atmosphere. Russ. Meteorol. Hydrol. 46, 762–767 (2021). https://doi.org/10.3103/S1068373921110054
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068373921110054