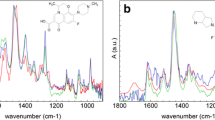
Abstract
We study the effect of the nature of the substituent in the β-cyclodextrin derivative on the physicochemical properties of the antibacterial drug ciprofloxacin (CF). CF can form guest–host inclusion complexes with β-cyclodextrin derivatives, characterized by dissociation constants (Kdis) in the range of 10–3 to 10–4 M. A ligand with a small polar uncharged substituent (2-hydroxypropyl) form of the most stable complex, which also helps to increase the solubility of CF by 20 and 64% in buffer systems having a pH of 4.0 and 7.4, respectively. The inclusion of CF in a complex with Kdis = 10–3 M or lower contributes to a slower release of the drug. The physicochemical properties of the obtained complexes allow develo** a highly effective formulation of the drug.





Similar content being viewed by others
REFERENCES
Davis, R., Markham, A., and Balfour, J.A., Drugs, 1996, vol. 51, no. 6, p. 1019.
Campoli-Richards, D.M., Monk, J.P., and Price, A., Drugs, 1988, vol. 35, p. 373.
Wolfson, J.S. and Hooper, D.C., Clin. Microbiol. Rev., 1989, vol. 2, no. 4, p. 378.
Fish, D.N., Pharmacotherapy, 2001, vol. 21, no. 10 P.
Ahmed, A.I.A., van der Heijden, F.M.M.A., van den Berkmortel, H., et al., Gen. Hosp. Psychiatry, 2011, vol. 33, no. 1, p. 82.e5.
Domagala, J.M., J. Antimicrob. Chemother., 1994, vol. 33, p. 685.
Zhanel, G.G., Ennis, K., Vercaigne, L., et al., Drugs, 2002, vol. 62, no. 1, p. 13.
Schacht, P., Arcieri, G., and Hullmann, R., Am. J. Med., 1989, vol. 87.
Davis, M.E. and Brewster, M.E., Nat. Rev. Drug Discovery, 2004, vol. 3, no. 12, p. 1023.
Raut, S.Y., Manne, A.S.N., Kalthur, G., et al., Curr. Pharm. Des., 2019, vol. 25, no. 4, p. 444.
Mohanty, J., Bhasikuttan, A.C., Nau, W.M., et al., J. Phys. Chem. B, 2006, vol. 110, no. 10, p. 5132.
Deygen, I.M., Egorov, A.M., and Kudryashova, E.V., Moscow Univ. Chem. Bull. (Engl. Transl.), 2016, vol. 71, no. 1, p. 387.
Koester, L.S., Guterres, S.S., Le Roch, M., et al., Drug Dev. Ind. Pharm., 2001, vol. 27, no. 6, p. 533.
Dsugi, N.F.A. and Elbashir, A.A., Spectrochim. Acta, Part A, 2015, vol. 137, p. 804.
Le-Deygen, I.M., Skuredina, A.A., Uporov, I.V., et al., Anal. Bioanal. Chem., 2017, vol. 409, no. 27, p. 6451.
Bobrovnik, S.A., J. Biochem. Biophys. Methods, 2004, vol. 93, no. 10, p. 2585.
Barri, T., Trtić-Petrović, T., Karlsson, M., et al., J. Pharm. Biomed. Anal., 2008, vol. 48, no. 1, p. 49.
Crupi, V., Ficarra, R., Guardo, M., et al., J. Pharm. Biomed. Anal., 2007, vol. 44, no. 1, p. 110.
Goswami, S. and Sarkar, M., New J. Chem., 2018, vol. 42, no. 18, p. 15146.
Hasanvand, E. and Rafe, A., Int. J. Biol. Macromol., 2019, vol. 131, p. 60.
Li, Z., Hong, H., Liao, L., et al., Colloids Surf., B, 2011, vol. 88, no. 1, p. 339.
Loftsson, T. and Petersen, D.S., Drug Dev. Ind. Pharm., 1998, vol. 24, no. 4, p. 365.
Van Doorslaer, X., Dewulf, J., van Langenhove, H., et al., Sci. Total Environ., 2014, vols. 500–501, p. 250.
Langlois, M.H., Montagut, M., Dubost, J.P., and Grellet, J., Eur. J. Pharm. Biopharm. Anal., 2005, vol. 37, p. 389.
Skuredina, A.A., Le-Deygen, I.M., and Kudryashova, E.V., Colloid J., 2018, vol. 80, no. 3, p. 312.
Tóth, G., Jánoska, Á., Völgyi, G., et al., J. Inclusion Phenom. Macrocyclic Chem., 2013, vol. 77, nos. 1–4, p. 291.
Misiuk, W. and Jozefowicz, M., J. Mol. Liq., 2015, vol. 202, p. 101.
Skuredina, A.A., Le-Deygen, I.M., Uporov, I.V., et al., Colloid J., 2017, vol. 79, no. 5, p. 668.
Dorofeev, V.L., Pharm. Chem. J., 2004, vol. 38, no. 12, p. 693.
Sukhoverkov, K.V., Le-Deygen, I.M., Egorov, A.M., et al., Russ. J. Phys. Chem. B, 2018, vol. 12, no. 7, p. 1193.
Yu, X., Zipp, G.L., and Ray Davidson, G.W., Pharm. Res., 1994, vol. 11, no. 4, p. 522.
Sukhoverkov, K.V., et al., Sverkhkrit. Flyuidy,Teor. Prakt., 2017, vol. 12, no. 4, p. 66.
ACKNOWLEDGMENTS
This work was supported by the UMNIK program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by O. Zhukova
About this article
Cite this article
Skuredina, A.A., Kopnova, T.Y., Le-Deygen, I.M. et al. Physical and Chemical Properties of the Guest–Host Inclusion Complexes of Cyprofloxacin with β-Cyclodextrin Derivatives. Moscow Univ. Chem. Bull. 75, 218–224 (2020). https://doi.org/10.3103/S0027131420040069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0027131420040069