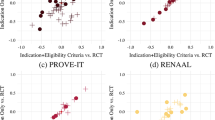
Abstract
Randomized controlled trials provide unbiased estimates of treatment effect through reducing the attribution of observed differences in outcome to the play of chance or the treatment of interest. Issues in the design of randomized trials may lead to a dilution or magnification of treatment effect when compared with clinical practice. The intention-to-treat principle will lead to dilution in the treatment effect, while the selective recruitment of adherent and motivated subjects may lead to enhanced treatment effect estimates. Observational data, on the other hand, may reflect clinical practice but cannot provide unbiased estimates of treatment effect. Health policy may be informed by the combination of randomized and observational data in decision-analytic models.

Similar content being viewed by others
References
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline: guideline for good clinical practice [E6 (R1)], 1996 [online]. Available from URL: http://www.ich.org/LOB/media/MEDIA482.pdf [Accessed 2008 May 21]
The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325 (5): 293–302
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline: statistical principles for clinical trials [E9], 1998 [online]. Available from URL: http://www.ich.org/LOB/media/MEDIA485.pdf [Accessed 2008 May 21]
Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetologia 2006; 49: 1711–21
Calvert MJ, McManus R, Freemantle N. The management of people with type 2 diabetes with multiple oral hypoglycaemic agents or insulin in primary care: retrospective cohort study. Br J Gen Pract 2007; 57: 455–60
Del Prato S, Blonde L, Martinez L, et al. The effect of the availability of inhaled insulin on glycaemic control in patients with type 2 diabetes failing on oral therapy: the evaluation of Exubera as a therapeutic option on insulin initiation and improvement in glycaemic control in clinical practice (EXPERIENCE) trial. Diabet Med 2008; 25 (6): 662–70
Freemantle N, Mathieu C, Hauner H. Replicating the PROactive trial of pioglitazone in subjects with diabetes and raised cardiovascular risk with the EAGLE model. ADA; 2006 Jun 9–13; Washington, DC. Diabetes 2006; 55 Suppl. 1: A112
Sculpher MJ, Pang FS, Manca A, et al. Generalisability in economic evaluation studies in healthcare: a review and case studies. Health Technol Assess 2004; 8: 1–192
Geddes JR, Freemantle N, Harrison P, et al., for the National Schizophrenia Guideline Development Group. Atypical antipsychotics in the treatment of schizophrenia: systematic review and meta-regression. BMJ 2000; 321: 1371–6
R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2008 [online]. Available from URL: http://www.r-project.org [Accessed 2008 Dec 2]
Acknowledgements
This article was funded by SA GmbH. Nick Freemantle has received funding for research, travel and consulting from Sanofi-Aventis and a number of other pharmaceutical and device companies. Franz Hessel is an employee of Sanofi-Aventis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freemantle, N., Hessel, F. The Applicability and Generalizability of Findings from Clinical Trials for Health-Policy Decisions. Pharmacoeconomics 27, 5–10 (2009). https://doi.org/10.2165/00019053-200927010-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200927010-00002