Abstract
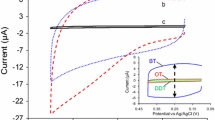
Nickel hexacyanoferrate (NiHCF) film was prepared and characterized on gold and thiol self-assembled monolayers (SAMs)-modified gold electrodes. It was found that the film exhibited some different electrochemical characteristics compared with that found on a carbon electrode. In the presence of K+, the film exhibited a redox peak at about 0.5 V. The peak potential shifted linearly with the K+ concentration over the range of about 0.1 mM–0.1 M with slopes of 54–60 mV per log[K+]. However, in solutions containing Na+, Li+ or NH4+ ion the film did not generate well-defined peaks, or even a visible redox peak. Therefore, the film showed a selective potential response to K+. The voltammetric behavior of NiHCF film varied with thiols, the preparation procedure and the solution pH. Under certain conditions, the characteristics of the film could be improved to some extent.
Similar content being viewed by others
References
V. D. Neff, J. Electrochem. Soc., 1978, 125, 886.
D. Ellis, M. Eckhoff, and V. D. Neff, J. Phys. Chem., 1981, 85, 1225.
K. Itaya, Uchida, and V. D. Neff, Acc. Chem. Res., 1986, 19, 162.
K. Itaya, H. Akahoshi, and S. Toshima, J. Appl. Phys., 1982, 53, 804.
K. Itaya, T. Ataka, S. Toshima, and T. Shinohara, J. Am. Chem. Soc., 1982, 104, 4767.
J. Joseph, H. Gomathi, and G. P. Rao, J. Electroanal. Chem., 1997, 431, 231.
S. Bharathi, J. Joseph, D. Jeyakumar, and G. P. Rao, J. Electroanal. Chem., 1991, 319, 341.
P. J. Kulesza, J. Electroanal. Chem., 1987, 220, 295.
K. Ogura and M. Kaneko, J. Molec. Catal., 1985, 31, 49.
V. D. Neff, J. Electrochem. Soc., 1985, 132, 1382.
S. J. Dong and B. Feng, J. Electroanal. Chem., 1986, 210, 31.
P. J. Kulesza, T. Jedral, and Z. Galus, Electrochim. Acta, 1989, 34, 851.
B. D. Humphrey, S. Sinha, and A. B. Bocarsly, J. Phys. Chem., 1987, 91, 586.
P. J. Kulesza, K. Brajter, and E. Dabek Zlotorzynska, Anal. Chem., 1987, 59, 2776.
J. Joseph, H. Gomathi, and G. P. Rao, Electrochim. Acta, 1991, 36, 1537.
A. Ulman, “An Introduction to Ultrathin Organic Films”, 1991, Academic Press, Boston.
A. Ulman, Chem. Rev., 1996, 96, 1533.
S. Berchmans, S. Arivukkodi, and V. Yegharaman, Electrochem. Commu., 2000, 2, 226.
J. Justin Gooding, L. Pugliano, D. Brynn Hibbert, and P. Erokhin, Electrochem. Commun., 2000, 2, 217.
J. Wang, B-Z. Zeng, C. Feng, and X-Y. Zhou, Anal. Sci., 2000, 16, 457.
C. A. Widrig, C. A. Alves, and M. D. Porter, J. Am. Chem. Soc., 1991, 113, 2805.
C. A. Widrig, C. Chung, and M. D. Porter, J. Electroanal. Chem., 1991, 310, 335.
M. G. Samant, C. A. Brown, and J. G. Gordon, Langmuir, 1991, 7, 437.
J. Wang, B-Z. Zeng, C. Feng, and X-Y. Zhou, Electroanalysis, 1999, 11, 1345.
J. Wang, B-Z. Zeng, C. Feng, and X-Y. Zhou, Electroanalysis, 2000, 12, 763.
J. Joseph, H. Gomathi, and G. Prabhakara Rao, Bull. Electrochem., 1990, 6, 170.
I. V. Tananaev, G. B. Seifer, Yu, Ya. Kharitonov, V. G Kuznetsov, and A. P. Korol’Kov, Ferrocyanide Chemisty, 1971, Nauka, Moscow.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zeng, B., Zhao, F. & Ding, X. Electrochemical Characteristics of Thin Nickel Hexacyanoferrate Films Formed on Gold and Thiol Self-Assembled Monolayers Modified Gold Electrodes. ANAL. SCI. 17, 259–264 (2001). https://doi.org/10.2116/analsci.17.259
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.17.259