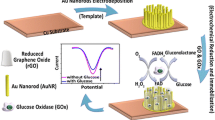
Abstract
This study effectively utilizes ion-sensitive field-effect transistor (ISFET) technology to detect enzymatic oxidation reactions. The research presents a method for immobilizing glucose oxidase (GOD) enzymes by activating groups that covalently attach to magnetite nanoparticles coated with a silicon oxide containing carboxyl groups (F-NPs). Amperometry techniques are utilized to detect glucose concentrations on the ISFET gate area through incubation with carbodiimides (EDC) and succinimides (NHS). The ISFET device comprises the organic semiconductors (P3HT/PC71BM) on an indium tin oxide (ITO) coated polyethylene terephthalate (PET) substrate for source and drain electrodes. A PET-ITO gate electrode improves the gate configuration by utilizing dual gates: one on the semiconductor layer and another on a laser-induced graphene (LIG) pattern, interconnected for immobilization platform (PI) which can detect changes in solution load different glucose levels. IV saturation curves display a continual ISFET drain current decrease with increasing glucose concentration, confirming efficacy as a glucose sensor.
Graphical abstract





Similar content being viewed by others
Data availability
Experiments and measurements were carried out in two teaching laboratories: ESFM and CNMN, which are both affiliated with the Instituto Politecnico Nacional. The following personnel conducted the measurements: M. Sc. Luis Alberto Moreno Ruiz carried out the FTIR spectroscopy, the TEM and SEM analysis were performed Ph.D Hugo Martínez Gutierrez and Ph.D Nicolas Cayetano Castro. For access to the data produced or examined in this study, please consult the responsible persons upon reasonable request. Additionally, for comprehensive details about the processes of synthesis, characterization, and device construction, please refer to the postgraduate thesis repository. The study will be entitled “Funcionalización de nanopartículas magneticas para la inmobilización de antígenos” and will be part of the PhD program in Nanosciences and Micro-Nanotechnologies at the ENCB-IPN, with expected availability in June 2024.
References
«Diabetes». Accedido: 25 de agosto de 2023. [En línea]. Disponible en: https://www.who.int/es/news-room/fact-sheets/detail/diabetes
K.-Y. Park, S.-B. Choi, M. Lee, B.-K. Sohn, S.-Y. Choi, ISFET glucose sensor system with fast recovery characteristics by employing electrolysis. Sens. Actuators B 83(1–3), 90–97 (2002). https://doi.org/10.1016/S0925-4005(01)01049-8
S. Ayaz, A. Üzer, Y. Dilgin, M.R. Apak, Fabrication of a novel optical glucose biosensor using Copper(II) neocuproine as a chromogenic oxidant and glucose dehydrogenase-immobilized magnetite nanoparticles. ACS Omega 8(49), 47163–47172 (2023). https://doi.org/10.1021/acsomega.3c07181
H. Yang, Y. Zhu, Size dependence of SiO2 particles enhanced glucose biosensor. Talanta 68(3), 569–574 (2006). https://doi.org/10.1016/j.talanta.2005.04.057
L. Mei, Y. Yang, J. Li, S. Shang, X. Fu, A SiO2 hybrid enzyme-based biosensor with enhanced electrochemical stability for accuracy detection of glucose. Int. J. Anal. Chem. 2023, 1–8 (2023). https://doi.org/10.1155/2023/6620613
B.-K. Sohn, B.-W. Cho, C.-S. Kim, D.-H. Kwon, ISFET glucose and sucrose sensors by using platinum electrode and photo-crosslinkable polymers. Sens. Actuators B 41(1–3), 7–11 (1997). https://doi.org/10.1016/S0925-4005(97)80271-7
T.I. Shabatina, O.I. Vernaya, V.P. Shabatin, M.Y. Melnikov, Magnetic nanoparticles for biomedical purposes: modern trends and prospects. Magnetochemistry 6(3), 30 (2020). https://doi.org/10.3390/magnetochemistry6030030
C.T. Tracey et al., Hybrid cellulose nanocrystal/magnetite glucose biosensors. Carbohydr. Polym. 247, 116704 (2020). https://doi.org/10.1016/j.carbpol.2020.116704
J. Jaime, G. Rangel, A. Muñoz-Bonilla, A. Mayoral, P. Herrasti, Magnetite as a platform material in the detection of glucose, ethanol and cholesterol. Sens. Actuators B 238, 693–701 (2017). https://doi.org/10.1016/j.snb.2016.07.059
A.-G. Niculescu, C. Chircov, A.M. Grumezescu, Magnetite nanoparticles: synthesis methods—a comparative review. Methods 199, 16–27 (2022). https://doi.org/10.1016/j.ymeth.2021.04.018
S. Liu, B. Yu, S. Wang, Y. Shen, H. Cong, Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 281, 102165 (2020). https://doi.org/10.1016/j.cis.2020.102165
T. Radu, A. Petran, D. Olteanu, I. Baldea, M. Potara, R. Turcu, Evaluation of physico-chemical properties and biocompatibility of new surface functionalized Fe3O4 clusters of nanoparticles. Appl. Surf. Sci. 501, 144267 (2020). https://doi.org/10.1016/j.apsusc.2019.144267
A.K. Gupta, M. Gupta, Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26(18), 3995–4021 (2005). https://doi.org/10.1016/j.biomaterials.2004.10.012
R. Eivazzadeh-Keihan et al., Functionalized magnetic nanoparticles for the separation and purification of proteins and peptides. TrAC Trends Anal. Chem. 141, 116291 (2021). https://doi.org/10.1016/j.trac.2021.116291
S.K. Vashist, Comparison of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide based strategies to crosslink antibodies on amine-functionalized platforms for immunodiagnostic applications. Diagnostics 2(3), 23–33 (2012). https://doi.org/10.3390/diagnostics2030023
C. Haavik, S. Stølen, H. Fjellvåg, M. Hanfland, D. Häusermann, Equation of state of magnetite and its high-pressure modification: thermodynamics of the Fe-O system at high pressure. Am. Mineral. 85(3–4), 514–523 (2000). https://doi.org/10.2138/am-2000-0413
B.A. Wechsler, D.H. Lindsley, C.T. Prewitt, Crystal structure and cation distribution in titanomagnetites (Fe3-xTixO4). Am. Mineral. 69(7–8), 754–770 (1984)
L. Levien, C.T. Prewitt, D.J. Weidner, Structure and elastic properties of quartz at pressure. Am. Mineral. 65(9–10), 920–930 (1980)
M. J. Buerger, Mineralogy: Dana’s System of Mineralogy, ed. by C. Palache, H. Berman, C. Frondel. Seventh Edition, Volume I, Elements, Sulfides, Sulfosalts, Oxides. (Wiley, New York, vol. 101, no. 2634, 1945), pp. 650–652. https://doi.org/10.1126/science.101.2634.650
F.T. Johra, J.-W. Lee, W.-G. Jung, Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 20(5), 2883–2887 (2014). https://doi.org/10.1016/j.jiec.2013.11.022
M. Liu et al., Bimetallic AuPt/TiO2 catalysts for direct oxidation of glucose and gluconic acid to tartaric acid in the presence of molecular O2. ACS Catal. 10(19), 10932–10945 (2020). https://doi.org/10.1021/acscatal.0c02238
N.A. ElSayed et al., Glycemic targets: standards of care in diabetes. Diabetes Care 46(Suppl 1), S97–S110 (2022). https://doi.org/10.2337/dc23-S006
Acknowledgments
GLAB is grateful to Consejo Nacional de Humanidades Ciencias y Tecnologías (CONAHCyT) for its financial support throughout her adscription to the doctoral program of nanosciences. HMG and RGA are grateful to COFAA-IPN, EDD-IPN, and EDI-IPN for support through academic fellowships.
Funding
The Instituto Politécnico Nacional (IPN) and the Secretaría de Investigación and Posgrado (SIP) supplied financial support for this research through projects numbered 20231885 and 20231318.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. GLAB performed the synthesis, device construction and the electrical and morphological analysis. JPAG and RGA collected electrical data for the IV curves. HMG acquired micrographs of the materials. The first draft of the manuscript was written by GLAB, and all authors commented on earlier versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Araujo-Bernal, G.L., Aguilar-González, J.P., Martínez-Gutiérrez, H. et al. Use of laser-induced graphene and magnetite nanoparticles as anchors in electrochemical glucose detection devices. MRS Advances 9, 161–167 (2024). https://doi.org/10.1557/s43580-024-00832-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-024-00832-1