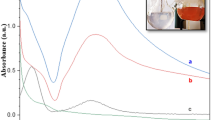
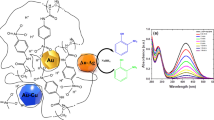
Abstract
The incorporation of phosphorous into metal nanoparticles can be an important way to change the material structure and catalytic properties. Colloidal synthesis, hot injection in particular, is well known for its versatility and can be an alternative approach for Janus nanomaterials’ synthesis. In this work, we show an example of the colloidal synthesis of metal–metal phosphides, by first synthesizing metal–metal nanoparticles followed by partial phosphidation, to convert Ag–Cu to Ag–Cu3P. We show that partial phosphidation of Ag–Cu nanoparticles can be achieved likewise, despite Ag incorporation increasing the stability. SEM, TEM, UV–Vis, and XPS were used to study the differences in structural, morphological' and chemical properties between Ag–Cu and Ag–Cu3P. The materials before and after phosphidation were used as electrocatalysts for hydrogen evolution in 0.1 M NaOH, the onset potential of Ag–Cu3P is 200 mV smaller than Ag–Cu, and the Tafel slope reduced from 218.3 mV/dec for Ag–Cu to 146.8 mV/dec for Ag–Cu3P. In summary, we showed an alternative approach to construct Ag–Cu3P nanoparticles. And this method might be used to construct other Janus metal–metal phosphide (A-BPx, A is the metal with higher redox potential, such as Ag that tends to retain after phosphidation, and B is the metal with lower redox potential, such as Cu and Ni, Fe, Co) nanoparticles and can be useful in energy conversion applications.
Graphical abstract





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
D. Wang, Y. Li, Bimetallic nanocrystals: liquid-phase synthesis and catalytic applications. Adv. Mater. 23(9), 1044–1060 (2011). https://doi.org/10.1002/adma.201003695
J. Qiu, Q.N. Nguyen, Z. Lyu, Q. Wang, Y. **a, Bimetallic Janus nanocrystals: syntheses and applications. Adv. Mater. 34(1), 2102591 (2022). https://doi.org/10.1002/adma.202102591
J. Huang, M. Mensi, E. Oveisi, V. Mantella, R. Buonsanti, Structural sensitivities in bimetallic catalysts for electrochemical CO2 reduction revealed by Ag–Cu nanodimers. J. Am. Chem. Soc. 141(6), 2490–2499 (2019). https://doi.org/10.1021/jacs.8b12381
C. Wadell, A. Yasuhara, T. Sannomiya, Asymmetric light absorption and radiation of Ag–Cu hybrid nanoparticles. J. Phys. Chem. C 121(48), 27029–27035 (2017). https://doi.org/10.1021/acs.jpcc.7b09926
S. Biswas, S. Pramanik, S. Mandal, S. Sarkar, S. Chaudhuri, S. De, Facile synthesis of asymmetric patchy Janus Ag/Cu particles and study of their antifungal activity. Front. Mater. Sci. 14(1), 24–32 (2020). https://doi.org/10.1007/s11706-020-0496-6
S. Kaja, A. Nag, Bimetallic Ag–Cu Alloy microflowers as SERS substrates with single-molecule detection limit. Langmuir 37(44), 13027–13037 (2021). https://doi.org/10.1021/acs.langmuir.1c02119
L. Rahman, A. Shah, S.K. Lunsford, C. Han, M.N. Nadagouda, E. Sahle-Demessie, R. Qureshi, M.S. Khan, H.-B. Kraatz, D.D. Dionysiou, Monitoring of 2-butanone using a Ag–Cu bimetallic alloy nanoscale electrochemical sensor. RSC Adv. 5(55), 44427–44434 (2015). https://doi.org/10.1039/C5RA03633J
M.S. Seifner, M. Snellman, O.A. Makgae, K. Kumar, D. Jacobsson, M. Ek, K. Deppert, M.E. Messing, K.A. Dick, Interface dynamics in Ag–Cu3P nanoparticle heterostructures. J. Am. Chem. Soc. 144(1), 248–258 (2022). https://doi.org/10.1021/jacs.1c09179
S. Carenco, Y. Hu, I. Florea, O. Ersen, C. Boissière, N. Mézailles, C. Sanchez, Metal-dependent interplay between crystallization and phosphorus diffusion during the synthesis of metal phosphide nanoparticles. Chem. Mater. 24(21), 4134–4145 (2012). https://doi.org/10.1021/cm3022243
E.A. Hernández-Pagán, R.W. Lord, J.M. Veglak, R.E. Schaak, Incorporation of metal phosphide domains into colloidal hybrid nanoparticles. Inorg. Chem. 60(7), 4278–4290 (2021). https://doi.org/10.1021/acs.inorgchem.0c03826
L. De Trizio, A. Figuerola, L. Manna, A. Genovese, C. George, R. Brescia, Z. Saghi, R. Simonutti, M. Van Huis, A. Falqui, Size-tunable, hexagonal plate-like Cu3P and Janus-like Cu–Cu3P nanocrystals. ACS Nano 6(1), 32–41 (2012). https://doi.org/10.1021/nn203702r
R. Wang, X.-Y. Dong, J. Du, J.-Y. Zhao, S.-Q. Zang, MOF-derived bifunctional Cu3P nanoparticles coated by a N, P-codoped carbon shell for hydrogen evolution and oxygen reduction. Adv. Mater. 30(6), 1703711 (2018). https://doi.org/10.1002/adma.201703711
H. Ahmad, A. Rauf, S. Muhammad, Theoretical investigation of the optoelectronic response of highly correlated Cu3P photocatalyst. RSC Adv. 12(32), 20721–20726 (2022). https://doi.org/10.1039/D2RA02472A
A. Crovetto, T. Unold, A. Zakutayev, Is Cu3–XP a semiconductor, a metal, or a semimetal? Chem. Mater. 35(3), 1259–1272 (2023). https://doi.org/10.1021/acs.chemmater.2c03283
P. Losch, W. Huang, E.D. Goodman, C.J. Wrasman, A. Holm, A.R. Riscoe, J.A. Schwalbe, M. Cargnello, Colloidal nanocrystals for heterogeneous catalysis. Nano Today 24, 15–47 (2019). https://doi.org/10.1016/j.nantod.2018.12.002
J.V.I. Timonen, E.T. Seppälä, O. Ikkala, R.H.A. Ras, From hot-injection synthesis to heating-up synthesis of cobalt nanoparticles: observation of kinetically controllable nucleation. Angew. Chem. Int. Ed. 50(9), 2080–2084 (2011). https://doi.org/10.1002/anie.201005600
A.E. Henkes, R.E. Schaak, Trioctylphosphine: a general phosphorus source for the low-temperature conversion of metals into metal phosphides. Chem. Mater. 19(17), 4234–4242 (2007). https://doi.org/10.1021/cm071021w
X. Zhao, Q. Di, M. Li, Q. Yang, Z. Zhang, X. Guo, X. Fan, K. Deng, W. Chen, J. Zhang, J. Fang, Z. Quan, Generalized synthesis of uniform metal nanoparticles assisted with tungsten hexacarbonyl. Chem. Mater. 31(12), 4325–4329 (2019). https://doi.org/10.1021/acs.chemmater.9b00219
C.F. Macrae, P.R. Edgington, P. McCabe, E. Pidcock, G.P. Shields, R. Taylor, M. Towler, J. van de Streek, Mercury: visualization and analysis of crystal structures. J. Appl. Crystallogr. 39(3), 453–457 (2006). https://doi.org/10.1107/S002188980600731X
W.T. Osowiecki, X. Ye, P. Satish, K.C. Bustillo, E.L. Clark, A.P. Alivisatos, Tailoring morphology of Cu–Ag nanocrescents and core-shell nanocrystals guided by a thermodynamic model. J. Am. Chem. Soc. 140(27), 8569–8577 (2018). https://doi.org/10.1021/jacs.8b04558
S.I. Bogatyrenko, A.P. Kryshtal, A. Kruk, Effect of size on the formation of solid solutions in Ag–Cu nanoparticles. J. Phys. Chem. C 127(5), 2569–2580 (2023). https://doi.org/10.1021/acs.jpcc.2c07132
T. Sun, Y. Wang, W. Yu, Y. Wang, Z. Dai, Z. Liu, B.N. Shivananju, Y. Zhang, K. Fu, B. Shabbir, W. Ma, S. Li, Q. Bao, Flexible broadband graphene photodetectors enhanced by plasmonic Cu3−xP colloidal nanocrystals. Small 13(42), 1701881 (2017). https://doi.org/10.1002/smll.201701881
M. Hlaing, B. Gebear-Eigzabher, A. Roa, A. Marcano, D. Radu, C.-Y. Lai, Absorption and scattering cross-section extinction values of silver nanoparticles. Opt. Mater. 58, 439–444 (2016). https://doi.org/10.1016/j.optmat.2016.06.013
J. Lin, C. Zeng, X. Lin, C. Xu, C.-Y. Su, CNT-assembled octahedron carbon-encapsulated Cu3P/Cu heterostructure by in situ MOF-derived engineering for superior lithium storage: investigations by experimental implementation and first-principles calculation. Adv. Sci. 7(14), 2000736 (2020). https://doi.org/10.1002/advs.202000736
H. Du, R.-M. Kong, X. Guo, F. Qu, J. Li, Recent progress in transition metal phosphides with enhanced electrocatalysis for hydrogen evolution. Nanoscale 10(46), 21617–21624 (2018). https://doi.org/10.1039/C8NR07891B
J.K. Nørskov, T. Bligaard, A. Logadottir, J.R. Kitchin, J.G. Chen, S. Pandelov, U. Stimming, Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152(3), J23 (2005). https://doi.org/10.1149/1.1856988
W. Sheng, M. Myint, J.G. Chen, Y. Yan, Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces. Energy Environ. Sci. 6(5), 1509–1512 (2013). https://doi.org/10.1039/C3EE00045A
J.R. Lombardi, R.L. Birke, A unified approach to surface-enhanced raman spectroscopy. J. Phys. Chem. C 112(14), 5605–5617 (2008). https://doi.org/10.1021/jp800167v
R.F. Zhang, X.F. Kong, H.T. Wang, S.H. Zhang, D. Legut, S.H. Sheng, S. Srinivasan, K. Rajan, T.C. Germann, An informatics guided classification of miscible and immiscible binary alloy systems. Sci. Rep. 7(1), 9577 (2017). https://doi.org/10.1038/s41598-017-09704-1
Acknowledgments
AL wishes to acknowledge financial support from the Center for Functional Materials (CFM) and the Center for Energy, Environment and Sustainability at Wake Forest University. Some of the characterizations described in this manuscript was performed in part at the Joint School of Nanoscience and Nanoengineering (JSNN), a member of the National Nanotechnology Coordinated Infrastructure (NNCI), which is supported by the National Science Foundation (Grant ECCS-2025462). The authors would like to thank Dr. Gayani Pathiraja for performing the TEM and STEM analysis and help from Mr. Kyle Williamson, Dr. Shobha Mantripragada from JSNN, and Dr. Chaochao Dun from Molecular Foundry for the XPS data measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, X., Lachgar, A. Metal–metal phosphide synthesis: Selective phosphidation of Ag–Cu nanocrystals. MRS Advances 8, 1010–1016 (2023). https://doi.org/10.1557/s43580-023-00604-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-023-00604-3