Abstract
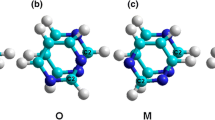
Single-walled carbon nanotube (SWNT) radical anions will react with tetrahydrofuran and generate ethylene, enolates, and a partially hydrogenated nanotube backbone. The experimental evidence suggests that there are sp3 C-H binding interactions. The total gravimetric content of hydrogen on a sample averages from 3.5% to 3.9% w/w, about four times the total amount observed for nanotubes hydrogenated via traditional Birch reduction reactions. Furthermore, the hydrogen desorbs at temperatures up to 400 °C less than those observed for the hydrogenated SWNTs formed after the Birch reduction. Finally, the first room temperature electron spin resonance spectrum of a nanotube radical ion is also reported.





Similar content being viewed by others
References
S. Pekker, J.-P. Salvetat, E. Jakab, J.-M. Bonard, and L. Forro: Hydrogenation of carbon nanotubes and graphite in liquid ammonia. J. Phys. Chem. B 105, 7938 (2001).
F. Borondics, M. Bokor, P. Matus, K. Tompa, S. Pekker, and E. Jakab: Reductive functionalization of carbon nanotubes. Fullerenes Nanotubes Carbon Nanostruct. 13, 375 (2005).
F. Borondics, E. Jakab, and S. Pekker: Functionalization of carbon nanotubes via dissolving metal reductions. J. Nanosci. Nanotechnol. 7, 1551 (2007).
A. Penicaud, P. Poulin, A. Derre, E. Anglaret, and P. Petit: Spontaneous dissolution of a single-wall carbon nanotube salt. J. Am. Chem. Soc. 127, 8 (2004).
F. Liang, L.B. Alemany, J.M. Beach, and W.E. Billups: Structure analyses of dodecylated single-walled carbon nanotubes. J. Am. Chem. Soc. 127, 13941 (2005).
F. Liang, J.M. Beach, K. Kobashi, A.K. Sadana, Y.I. Vega-Cantu, J.M. Tour, and W.E. Billups: In situ polymerization initiated by single-walled carbon nanotube salts. Chem. Mater. 18, 4764 (2006).
J. Chattopadhyay, F. de Jesus Cortez, S. Chakraborty, N.K.H. Slater, and W.E. Billups: Synthesis of water-soluble PEGylated single-walled carbon nanotubes. Chem. Mater. 18, 5864 (2006).
J. Chattopadhyay, A.K. Sadana, F. Liang, J.M. Beach, Y. **ao, R.H. Hauge, and W.E. Billups: Carbon nanotube salts. Arylation of single-wall carbon nanotubes. Org. Lett. 7, 4067 (2005).
J.A. Teprovich, M.S. Wellons, R. Lascola, S.-J. Hwang, P.A. Ward, R.N. Compton, and R. Zidan: Synthesis and characterization of a lithium-doped fullerane (Lix-C60-Hy) for reversible hydrogen storage. Nano Lett. 12, 582 (2011).
R.B. Bates, L.M. Kropskai, and D.E. Potter: Cycloreversions of anions from tetrahydrofurans. A convenient synthesis of lithium enolates of aldehydes. J. Org. Chem. 37, 560 (1972).
J. Carnahan and W.D. Closson: Reaction of naphthalene dianions with tetrahydrofuran and ethylene. J. Org. Chem. 37, 4469 (1972).
J. Clayden and S.A. Yasin: Pathways for decomposition of THF by organolithiums: The role of HMPA. New J. Chem. 26, 191 (2002).
M.J. Heben, A.C. Dillon, K.E.H. Gilbert, P.A. Parilla, T. Gennett, J.L. Alleman, G.L. Hornyak, and K.M. Jones: Assessing the hydrogen adsorption capacity of single-wall carbon nanotube/metal composites, in Proceedings of the First International Workshop on Hydrogen in Materials and Vacuum Systems, edited by G.R. Myneni and S. Chattopadhyay (AIP Conf. Proc. 671, New York, NY, 2003) p. 77.
A.C. Dillon, T. Gennett, K.M. Jones, J.L. Alleman, P.A. Parilla, and M.J. Heben: A simple and complete purification of single-walled carbon nanotube materials. Adv. Mater. 11, 1354 (1999).
T. Gennett, A.C. Dillon, J.L. Alleman, K.M. Jones, F.S. Hasoon, and M.J. Heben: Formation of single-wall carbon nanotube super-bundles. Chem. Mater. 12, 599 (2000).
G.R. Stevenson, C.R. Wiedrlch, S.S. Zlgler, L. Echegoyen, and R. Maldonado: The thermochemistry of solid naphthalene anion salts and their interaction with water. J. Phys. Chem 87, 4995 (1983).
H.C. Wang, C. Levin, and M. Szwarc: Comment on the communication: Production of hydrogen from interaction of an anion radical and water. J. Am. Chem. Soc. 100, 3969 (1978).
C.J. Curtis, T. Gennett, C. Engtrakul, K. O’Neill, J.E. Ellis, and M.J. Heben: Mechanism of hydrogen storage on reduced carbon single-walled nanotubes. in The Hydrogen Economy, edited by A. Dillon, C. Moen, B. Choudhury, and J. Keller (Mater. Res. Soc. Symp. Proc. 1098, Warrendale, PA, 2008). 1098-HH04-04.
C.W. Bauschlicher: High coverages of hydrogen on a (10,0) carbon nanotube. Nano Lett. 1, 223 (2001).
K.A. Park, K. Seo, and Y.H. Lee: Adsorption of atomic hydrogen on single-walled carbon nanotubes. J. Phys. Chem. B 109, 8967 (2005).
A.D. Martino, R. Egger, K. Hallberg, and C.A. Balseiro: Spin-orbit coupling and electron spin resonance theory for carbon nanotubes. Phys. Rev. Lett. 88, 206402 (2002).
J.P. Salvetat, T. Fehér, C. L’Huillier, F. Beuneu, and L. Forró: Anomalous electron spin resonance behavior of single-walled carbon nanotubes. Phys. Rev. B 72, 075440 (2005).
S.V. Rosokha and J.K. Kochi: The question of aromaticity in open-shell cations and anions as ion-radical offsprings of polycyclic aromatic and antiaromatic hydrocarbons. J. Org. Chem. 71, 9357 (2006).
A.C. Dillon, M. Yudasaka, and M.S. Dresselhaus: Employing Raman spectroscopy to qualitatively evaluate the purity of carbon single-wall nanotube materials. J. Nanosci. Nanotechnol. 4, 691 (2004).
A. Claye, S. Rahman, J.E. Fischer, A. Sirenko, G.U. Sumanasekera, and P.C. Eklund: In situ Raman scattering studies of alkali-doped single wall carbon nanotubes. Chem. Phys. Lett. 333, 16 (2001).
S. Gupta, M. Hughes, A.H. Windle, and J. Robertson: Charge transfer in carbon nanotube actuators investigated using in situ Raman spectroscopy. J. Appl. Phys. 95, 2038 (2004).
A.M. Rao, P.C. Eklund, S. Bandow, A. Thess, and R.E. Smalley: Evidence for charge transfer in doped carbon nanotube bundles from Raman scattering. Nature 388, 257 (1997).
G. Zhang, P. Qi, X. Wang, Y. Lu, D. Mann, X. Li, and H. Dai: Hydrogenation and hydrocarbonation and etching of single-walled carbon nanotubes. J. Am. Chem. Soc. 128, 6026 (2006).
A.C. Dillon, P.A. Parilla, J.L. Alleman, T. Gennett, K.M. Jones, and M.J. Heben: Systematic inclusion of defects in pure carbon single-wall nanotubes and their effect on the Raman D-band. Chem. Phys. Lett. 401, 522 (2005).
B.N. Khare, M. Meyyappan, A.M. Cassell, C.V. Nguyen, and J. Han: Functionalization of carbon nanotubes using atomic hydrogen from a glow discharge. Nano Lett. 2, 73 (2002).
B.N. Khare, M. Meyyappan, J. Kralj, P. Wilhite, M. Sisay, H. Imanaka, J. Koehne, and C.W. Bauschlicher: A glow-discharge approach for functionalization of carbon nanotubes. Appl. Phys. Lett. 81, 5237 (2002).
G.P. Miller, J. Kintigh, E. Kim, P.F. Weck, S. Berber, and D. Tomanek: Hydrogenation of single-wall carbon nanotubes using polyamine reagents: Combined experimental and theoretical study. J. Am. Chem. Soc. 130, 2296 (2008).
E. König, H. Musso, and U.-I. Zahorszky: Phenyldiimine in the reduction of diazonium salts by sodium tetrahydridoborate. Angew. Chem. Int. Ed. 11, 45 (1972).
L. Edmana, A. Herold, P. Jacobsson, M. Lelaurain, E. McRaeb, and B. Sundqvista: Sodium-sodium halide co-intercalated graphite: Chemistry, structure and electrical transport. J. Phys. Chem. Solids 60, 475 (1999).
A. Metrot, D. Guerard, D. Billaud, and A. Herold: New results about the sodium-graphite system. Synth. Met. 1, 363 (1980).
S. Pruvost, C. Herold, A. Herold, and P. Lagrange: Co-intercalation into graphite of lithium and sodium with an alkaline earth metal. Carbon 42, 1825 (2004).
Acknowledgments
Funding for this effort was provided by the U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy within the Center of Excellence on Hydrogen Sorption Materials as part of DOE’s National Hydrogen Storage Grand Challenge and by the Office of Science, Basic Energy Sciences, Materials Science and Engineering under subcontract DE-AC36-99GO10337 to NREL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Address all correspondence to this author
Rights and permissions
About this article
Cite this article
Engtrakul, C., Curtis, C.J., Ellis, J.E. et al. Reactions and reversible hydrogenation of single-walled carbon nanotube anions. Journal of Materials Research 27, 2806–2811 (2012). https://doi.org/10.1557/jmr.2012.298
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2012.298