Abstract
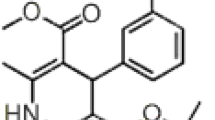
The objective of the current study was the development and subsequent validation of a simple, sensitive, precise and stability-indicating reversed-phase HPLC method for the determination of ciprofloxacin HCl in pharmaceutical dosage forms in the presence of its potential impurities. The chromatographic separation of ciprofloxacin HCl and its related compounds was achieved on an Inertsil ODS3 column using UV detection. The optimized mobile phase consisted of phosphoric acid solution: acetonitril. The proposed method provided linear responses within the concentration range 250–750 μg mL−1 for ciprofloxacin HCl and 0.5–1.5 μg mL−1 for its related compounds. LOD and LOQ values for the active substance were 5.159 and 15.632 μg mL−1, respectively. Correlation coefficients (r) of the regression equations for the impurities were greater than 0.99 in all cases. The precision of the method was demonstrated using intra- and inter-day assay RSD% values which were less than 1% in all instances. No interference from any components of pharmaceutical dosage forms or degradation products was observed.



Similar content being viewed by others
References
Fass RJ (1983) Antimicrob Agents Chemother 24:568–574
Chin NX, Neu HC (1984) Antimicrob Agents Chemother 25:319–326
Wolfson JS, Hooper DC (1985) Antimicrob Agents Chemother 28:581–586
Campoli-Richards DM, Monk JP, Price A, Benfield P, Todd PA, Ward A (1988) Drugs 35:373–447
Goodman and Gilman’s (1996) The pharmacological basis of therapeutics, 9th edn. McGraw-Hill, New York, p 1065
Jehl F, Gallion C, Debs J, Brogard JM, Monteil H, Minck R (1985) J Chromatogr 339:347–357
Weber A, Chaffin D, Smith A, Opheim KE (1985) Antimicrob Agents Chemother 27:531–534
Vallee F, LeBel M, Bergeron MG (1986) Ther Drug Monit 8:340–345
Kamberi M, Tsutsumi K, Kotegawa T, Nakamura K, Nakano S (1998) Clin Chem 44:1251–1255
Maya MT, Goncalves NJ, Silva NB, Morais JA (2001) J Chromatogr B 755(1–2):305–309
Vybíralová Z, Nobilis M, Zoulova J, Květina J, Petr P (2005) J Pharm Biomed Anal 37:851–858
Imre S, Dogaru MT, Vari CE, Muntean T, Kelemen L (2003) J Pharm Biomed Anal 33:125–130
Liang H, Kays MB, Sowinski KM (2002) J Chromatogr B 772(1):53–63
Ballesteros O, Toro I, Sanz-Nebot V, Navalón A, Vílchez JL, Barbosa J (2003) J Chromatogr B 798:137–144
Ramos M, Aranda A, Garcia E, Reuvers T, Hooghuis H. (2003) J Chromatogr B 789(2):373–381
Vega E, Dabbene V, Nassetta M, Sola N (1999) J Pharm Biomed Anal 21:1003–1009
Thoppil SO, Amin PD (2000) J Pharm Biomed Anal 22:699–703
Novakovic J, Nesmerak K, Nova H, Filka K (2001) J Pharm Biomed Anal 25:957–964
Shervington LA, Abba M, Hussain B, Donnelly J (2005) J Pharm Biomed Anal 39:769–775
Lacroix PM, Curran NM, Sears RW (1996) J Pharm Biomed Anal 14:641–654
European Pharmacopoeia 4.6 (2004) 3969
Barbosa J, Bergés R, Sanz-Nebot V (1998) J Chromatogr A 823(1–2):411–422
ICH Q2A Text on Validation of Analytical Methods (1995) Definition and Terminology (International Conference on Harmonisation of Technical requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland)
ICH Q2B Validation of Analytical Procedures (1997) Methodology (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland)
Krull I, Swartz M (1999) LCGC North Am 17:244–246
Torniainen K, Tammilehto S, Ulvi V (1996) Int J Pharm 132:53–61
Torniainen K, Mattinen J, Askolin CP, Tammilehto S (1997) J Pharm Biomed Anal 15:887–894
Torniainen K, Askolin CP, Mattinen J (1997) J Pharm Biomed Anal 16:439–445
Acknowledgments
The authors greatly acknowledge Biofarma Pharmaceutical Industry Co. Inc., İstanbul, Turkey, for providing the gift samples of ciprofloxacin hydrochloride and its related compounds as well as Cipro® 500 mg film-coated tablet specimens and placebo powder mixture utilized throughout this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Application of Separation Techniques in Turkey
Rights and permissions
About this article
Cite this article
Aksoy, B., Küçükgüzel, İ. & Rollas, S. Development and Validation of a Stability-Indicating HPLC Method for Determination of Ciprofloxacin Hydrochloride and its Related Compounds in Film-Coated Tablets. Chroma 66 (Suppl 1), 57–63 (2007). https://doi.org/10.1365/s10337-007-0287-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-007-0287-6