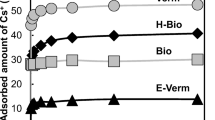
Abstract
The adsorption on montmorillonite of two monovalent organic cations, methylene blue (MB) and thioflavin T (TFT), was studied in four different situations: (1) separate adsorption of MB or TFT; (2) competitive adsorption of TFT and Cs; (3) competitive adsorption of the two organic cations from their equimolar solutions; and (4) adsorption of TFT on a clay whose cation-exchange capacity (CEC) had been previously saturated with MB. MB and TFT adsorbed to as much as 120% and 140% of the CEC, respectively. Cs did not appear to compete with TFT for the adsorption sites of the clay. TFT molecules adsorbed more strongly than those of MB and displaced them from the clay surface. A model was developed to evaluate the strength of the clay-organic cation interactions. The specific binding of the cations to the negatively charged surface, determined by solving the electrostatic equations, appears to account for adsorption exceeding the CEC and formation of positively charged complexes, which are due to non-coulombic interactions between the organic ligands.
The charge reversal predicted by the model beyond the CEC of the clay was confirmed by microelec-trophoretic experiments. Particles in a sample of montmorillonite loaded with 50 meq TFT/100 g clay moved to the positive electrode, whereas in samples containing the two dyes, MB and TFT, coadsorbed at a total concentration of 100–120 meq/100 g clay, the particles moved to the negative electrode. Binding coefficients describing the formation of neutral and charged complexes of TFT and the clay were larger than those for MB and the clay, thereby explaining the preferential adsorption of TFT observed experimentally. The binding coefficients for the formation of neutral complexes of either MB and TFT and the clay were more than six orders of magnitude larger than those previously reported for inorganic monovalent cations.
Similar content being viewed by others
References
Barrow, N. J., Bowden, J. W., Posner, A. M., and Quirk, J. P. (1980) An objective method for fitting models of ion adsorption on variable charge surfaces: Aust. J. Soil Res. 18, 37–47.
Bowden, J. W., Posner, A. M., and Quirk, J. P. (1977) Ionic adsorption on variable charge mineral surfaces. Theoretical charge development and titration curves: Aust. J. Soil Res. 15, 121–136.
Chu, C. H. and Johnson, L. J. (1979) Cation-exchange behavior of clays and synthetic aluminosilica gels: Clays & Clay Minerals 27, 87–90.
Davis, J. A., James, R. O., and Leckie, J. O. (1978) Surface ionization and complexation at the oxide/water interface. 1. Computation of electrical double layer properties in simple electrolytes: J. Colloid Interface Sci. 63, 480–499.
De, D. K., Das Kanungo, J. L., and Chakravarti, S. K. (1974) Interaction of crystal violet and malachite green with ben-tonite and their desorption by inorganic and surface active quaternary ammonium ions: Indian J. Chem. 12, 165–166.
Eisenberg, M., Gresalfy, T., Riccio, T., and McLaughlin, S. (1979) Adsorption of monovalent cations to bilayer membranes containing negative phospholipids: Biochemistry 18, 5213–5223.
Ghosal, D. N. and Mukherjee, S. K. (1972) Studies on the sorption and desorption of crystal violet on and from ben-tonite and kaolinite: J. Indian Chem. Soc. 49, 569–572.
Grauer, Z., Avnir, D., and Yariv, S. (1984) Adsorption characteristics of rhodamine 6G on montmorillonite and La-ponite, elucidated from electronic absorption and emission spectra: Can. J. Chem. 62, 1889–1894.
Kurland, R., Newton, C., Nir, S., and Papahadjopoulos, D. (1979) Specificity of Na+ binding to phosphatidylserine vesicles from a “Na NMR relaxation rate study: Biochim. Biophys. Acta 551, 137–147.
Margulies, L., Cohen, E., and Rozen, H. (1987) Photo-stabilization of bioresmethrin by organic cations on a clay surface: Pest. Sci. 18, 79–87.
Margulies, L. and Rozen, H. (1986) Adsorption of methyl green on montmorillonite: J. Molec. Structure 141, 219–226.
Margulies, L., Rozen, H., and Cohen, E. (1985) Energy transfer at the surface of clays and protection of pesticides from photodegradation: Nature 315, 658–659.
Margulies, L., Rozen, H., and Cohen, E. (1987) Photosta-bilization of a nitromethylene heterocycle on the surface of montmorillonite: Clays & Clay Minerals, 36, 159–164.
McBride, M. B., Pinnavaia, T. J., and Mortland, M. M. (1975a) Perturbation of structural Fe3+ in smectites by exchange ions: Clays & Clay Minerals 23, 103–107.
McBride, M. B., Pinnavaia, T. J., and Mortland, M. M. (1975b) Exchange ion positions in smectite: Effects on electron spin resonance of structural iron: Clays & Clay Minerals 23, 162–163.
Mortland, M. M. (1970) Clay-organic complexes and interactions: Adv. Agron. 22, 75–117.
Narine, D. R. and Guy, R. D. (1981) Interactions of some large organic cations with bentonite in dilute aqueous systems: Clays & Clay Minerals 29, 205–212.
Nir, S. (1984) A model for cation adsorption in closed systems: Application to calcium binding to phospholipid vesicles: J. Colloid Interface Sci. 102, 313–321.
Nir, S. (1986) Specific and non-specific cation adsorption to clays: Solution concentrations and surface potentials. Soil Sci. Soc. Amer. J. 50, 52–57.
Nir, S., Hirsch, D., Navrot, J., and Banin, A. (1986) Specific adsorption of lithium, sodium, potassium, cesium, and strontium to montmorillonite: Observations and predictions: Soil Sci. Soc. Amer. J. 50, 40–45.
Nir, S., Newton, C., and Papadhadjopoulos, D. (1978) Binding of cations to phosphatidylserine vesicles: Bioelectro-chem. Bioenerg. 5, 116–133.
Pham Thi Hang and Brindley, G. W. (1970) Methylene blue adsorption by clay minerals. Determination of surface areas and cation exchange capacities (Clay-organic studies XVIII): Clays & Clay Minerals 18, 203–212.
Shainberg, I. and Kemper, W. D. (1966) Hydration status of adsorbed cations: Soil Sci. Soc. Amer. Proc. 30, 707–713.
Theng, B. K. G. (1974) The Chemistry of Clay-Organic Reactions: Wiley, New York, 343 pp.
van Olphen, H. and Fripiat, J. J. (1979) Data Handbook for Clay Materials and Other Non-Metallic Minerals: Pergamon Press, Oxford, p. 19.
Venugopal, J. S. and Nair, M. M. (1974) Preferential dye sorption in clay minerals by acid treatment: Indian Mineral. 15, 23–27.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Margulies, L., Rozen, H. & Nir, S. Model for Competitive Adsorption of Organic Cations on Clays. Clays Clay Miner. 36, 270–276 (1988). https://doi.org/10.1346/CCMN.1988.0360309
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1988.0360309