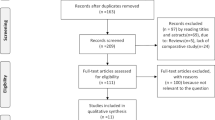
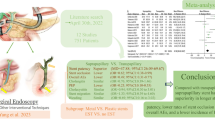
Abstract
Background
Optimal preoperative biliary drainage for patients with pancreatic cancer before pancreatoduodenectomy remains unclear. This study aimed to investigate the comparison of efficacy and safety between a metallic stent (MS) and a plastic stent (PS).
Methods
Comparative studies on the use of MS and PS for pancreatic cancer before pancreatoduodenectomy were systematically searched using the MEDLINE and Web of Science databases. Pre- and postoperative data also were extracted. Random-effects meta-analyses were performed to compare post-endoscopic retrograde cholangiopancreatography (ERCP) complications as well as intra- and postoperative outcomes between the two arms of the study, and pooled odds ratios (ORs) or mean differences (MDs) were calculated with 95 percent confidence intervals (CIs).
Results
The study analyzed 12 studies involving 683 patients. Insertion of MS was associated with a lower incidence of re-intervention (OR, 0.06; 95% CI 0.03–0.15; P < 0.001), increased post-ERCP adverse events (OR, 2.22; 95% CI 1.13–4.36; P = 0.02), and similar operation time (MD, 18.0 min; 95% CI –29.1 to 65.6 min; P = 0.46), amount of blood loss (MD, 43.0 ml; 95% CI –207.1 to 288.2 ml; P = 0.73), and surgical complication rate (OR, 0.78; 95% CI 0.53–1.15; P = 0.21). The cumulative stent patency rate after 3 months was higher in the MS group than in the PS group (70–100 % vs 30.0–45.0 %).
Conclusion
For biliary drainage in patients with pancreatic cancer during this era of multidisciplinary treatment, MS use might be the first choice because MS provides a more durable biliary drainage and a similar risk of postoperative outcomes compared with PS.




Similar content being viewed by others
Data Availability
PROSPERO 423627.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Patel SH, Katz MHG, Ahmad SA. The Landmark Series: preoperative therapy for pancreatic cancer. Ann Surg Oncol. 2021;28:4104–29.
Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10:10–27.
van Dam JL, Janssen QP, Besselink MG, et al. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomised controlled trials. Eur J Cancer. 2022;160:140–9.
Weber A, Mittermeyer T, Wagenpfeil S, Schmid RM, Prinz C. Self-expanding metal stents versus polyethylene stents for palliative treatment in patients with advanced pancreatic cancer. Pancreas. 2009;38:e7–12.
Hasegawa S, Endo I, Kubota K. Plastic or self-expandable metal stent: which is the most suitable for patients with pancreatic head cancer in the upcoming era of neoadjuvant chemotherapy? a review. Dig Endosc. 2021;34(2):297–306.
Cote GA, Kumar N, Ansstas M, et al. Risk of post-ERCP pancreatitis with placement of self-expandable metallic stents. Gastrointest Endosc. 2010;72:748–54.
Cao J, Peng C, Ding X, et al. Risk factors for post-ERCP cholecystitis: a single-center retrospective study. BMC Gastroenterol. 2018;18:128.
Isayama H, Yasuda I, Ryozawa S, et al. Results of a Japanese multicenter, randomized trial of endoscopic stenting for non-resectable pancreatic head cancer (JM-test): covered Wallstent versus DoubleLayer stent. Dig Endosc. 2011;23:310–5.
Vakil N. Expandable metal stents: principles and tissue responses. Gastrointest Endosc Clin North Am. 2011;21(351–7):vii.
Vakil N, Gross U, Bethge N. Human tissue responses to metal stents. Gastrointest Endosc Clin North Am. 1999;9:359–65.
Mirkin KA, Hollenbeak CS, Wong J. Time to surgery: a misguided quality metric in early-stage pancreatic cancer. J Gastrointest Surg. 2018;22:1365–75.
Decker C, Christein JD, Phadnis MA, Wilcox CM, Varadarajulu S. Biliary metal stents are superior to plastic stents for preoperative biliary decompression in pancreatic cancer. Surg Endos. 2011;25:2364–7.
Kubota K, Sato T, Watanabe S, et al. Covered self-expandable metal stent deployment promises safe neoadjuvant chemoradiation therapy in patients with borderline resectable pancreatic head cancer. Dig Endosc. 2014;26:77–86.
Tol JA, van Hooft JE, Timmer R, et al. Metal or plastic stents for preoperative biliary drainage in resectable pancreatic cancer. Gut. 2016;65:1981–7.
Tsuboi T, Sasaki T, Serikawa M, et al. Preoperative biliary drainage in cases of borderline resectable pancreatic cancer treated with neoadjuvant chemotherapy and surgery. Gastroenterol Res Pract. 2016;2016:7968201.
Nakamura K, Sho M, Akahori T, et al. A comparison between plastic and metallic biliary stent placement in patients receiving preoperative neoadjuvant chemoradiotherapy for resectable pancreatic cancer. World J Surg. 2019;43:642–8.
Kuwatani M, Nakamura T, Hayashi T, et al. Clinical outcomes of biliary drainage during a neoadjuvant therapy for pancreatic cancer: metal versus plastic stents. Gut Liver. 2020;14:269–73.
Hasegawa S, Kubota K, Yagi S, et al. Covered metallic stent placement for biliary drainage could be promising in the coming era of neoadjuvant chemo-radiation therapy for all pancreatic cancer. J Hepatobiliary Pancreat Sci. 2021;28(7):617–24.
Kobayashi K, Kobara H, Kamada H, et al. Comparison of plastic stent versus metal stent in preoperative biliary drainage for pancreatic head cancer with neoadjuvant chemoradiotherapy. J Hepatobiliary Pancreat Sci. 2021;28(10):856–63.
Tamura T, Itonaga M, Ashida R, et al. Covered self-expandable metal stents versus plastic stents for preoperative biliary drainage in patient receiving neo-adjuvant chemotherapy for borderline resectable pancreatic cancer: a prospective randomized study. Dig Endosc. 2021;33(7):1170–8.
Mandai K, Tsuchiya T, Kawakami H, et al. Fully covered metal stents vs plastic stents for preoperative biliary drainage in patients with resectable pancreatic cancer without neoadjuvant chemotherapy: a multicenter, prospective, randomized controlled trial. J Hepatobiliary Pancreat Sci. 2021;29(11):1185–94.
Ichikawa H, Iwashita T, Iwasa Y, et al. Covered self-expandable metallic stent versus plastic stent for preoperative endoscopic biliary drainage in patients with pancreatic cancer: a multi-center retrospective cohort study. Scand J Gastroenterol. 2021;57(4):493–500.
Kataoka F, Inoue D, Watanabe M, et al. Efficacy of 6-mm diameter fully covered self-expandable metallic stents in preoperative biliary drainage for pancreatic ductal adenocarcinoma. DEN Open. 2022;2:e55.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med. 2009;6:e1000100.
Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Systematic Rev. 2019;10:ED000142.
Irving JD, Adam A, Dick R, Dondelinger RF, Lunderquist A, Roche A. Gianturco expandable metallic biliary stents: results of a European clinical trial. Radiology. 1989;172:321–6.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A, Collaboration DESD. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020:962280219889080.
Marchet A, Mocellin S, Ambrosi A, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543–52.
Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–57.
Tachezy M, Gebauer F, Petersen C, et al. Sequential neoadjuvant chemoradiotherapy (CRT) followed by curative surgery vs. primary surgery alone for resectable, non-metastasized pancreatic adenocarcinoma: NEOPA: a randomized multicenter phase III study (NCT01900327, DRKS00003893, ISRCTN82191749). BMC Cancer. 2014;14:411.
Motoi F, Satoi S, Honda G, et al. A single-arm, phase II trial of neoadjuvant gemcitabine and S1 in patients with resectable and borderline resectable pancreatic adenocarcinoma: PREP-01 study. J Gastroenterol. 2019;54(2):194–203.
Ahmad SA, Duong M, Sohal DPS, et al. Surgical outcome results from SWOG S1505: a randomized clinical trial of mFOLFIRINOX versus gemcitabine/nab-paclitaxel for perioperative treatment of resectable pancreatic ductal adenocarcinoma. Ann Surg. 2020;272:481–6.
Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin. 2020;38:1763–73.
Janssen QP, van Dam JL, Bonsing BA, et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer. 2021;21:300.
Du J, Gao X, Zhang H, Wan Z, Yu H, Wang D. Stent selection in preoperative biliary drainage for patients with operable pancreatic cancer receiving neoadjuvant therapy: a meta-analysis and systematic review. Front Surg. 2022;9:875504.
Kumar N, Jena A, Sharma V, Shukla S, Shah J. Outcome of metal vs plastic stents for biliary obstruction in patients with pancreatic carcinoma undergoing neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci. 2023;30(4):419–28.
Swords DS, Zhang C, Presson AP, Firpo MA, Mulvihill SJ, Scaife CL. Association of time-to-surgery with outcomes in clinical stage I-II pancreatic adenocarcinoma treated with upfront surgery. Surgery. 2018;163:753–60.
Prat F, Chapat O, Ducot B, et al. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1–7.
Katsinelos P, Paikos D, Kountouras J, et al. Tannenbaum and metal stents in the palliative treatment of malignant distal bile duct obstruction: a comparative study of patency and cost effectiveness. Surg Endosc. 2006;20:1587–93.
Crippa S, Cirocchi R, Partelli S, et al. Systematic review and meta-analysis of metal versus plastic stents for preoperative biliary drainage in resectable periampullary or pancreatic head tumors. Eur J Surg Oncol. 2016;42:1278–85.
Ge PS, Hamerski CM, Watson RR, et al. Plastic biliary stent patency in patients with locally advanced pancreatic adenocarcinoma receiving downstaging chemotherapy. Gastrointest Endosc. 2015;81:360–6.
Kitano M, Yamashita Y, Tanaka K, et al. Covered self-expandable metal stents with an anti-migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: a randomized multicenter trial. Am J Gastroenterol. 2013;108:1713–22.
Gardner TB, Spangler CC, Byanova KL, et al. Cost-effectiveness and clinical efficacy of biliary stents in patients undergoing neoadjuvant therapy for pancreatic adenocarcinoma in a randomized controlled trial. Gastrointest Endosc. 2016;84:460–6.
Euscher ED, Marsh WL Jr, Lucas JG, Frankel WL. Histologic and immunohistochemical changes in the stented common bile duct. Appl Immunohistochem Mol Morphol. 2007;15:299–304.
Cavell LK, Allen PJ, Vinoya C, et al. Biliary self-expandable metal stents do not adversely affect pancreaticoduodenectomy. Am J Gastroenterol. 2013;108:1168–73.
Darnell EP, Wang TJ, Lumish MA, et al. Preoperative cholangitis is an independent risk factor for mortality in patients after pancreatoduodenectomy for pancreatic cancer. Am J Surg. 2021;221:134–40.
Kosaka H, Satoi S, Kono Y, et al. Estimation of the degree of surgical difficulty anticipated for pancreatoduodenectomy: preoperative and intraoperative factors. J Hepatobiliary Pancreat Sci. 2022;29(11):1166–74.
Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372–7.
National Comprehensive Cancer Network. Pancreatic Cancer, 2023. Retrieved7 September 2023 at https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
National Comprehensive Cancer Network. Biliary Tract Cancer. 2023. Retrieved 7 September 2023 at https://www.nccn.org/professionals/physician_gls/pdf/btc.pdf.
Mellado S, Vega EA, Abudalou M, et al. Trends in preoperative chemotherapy utilization for proximal pancreatic cancer: are we making progress? J Gastrointest Surg. 2022;26:1–7.
Acknowledgment
We thank Editage (http://www.editage.com) for English-language editing and reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
FIG. S1
Forest plot of studies examining ERCP-related adverse events. ERCP, endoscopic retrograde cholangiopancreatography. FIG. S2 Forest plot of studies examining postoperative findings. A Blood loss. B Operation time. C Surgical complication. D Mortality. FIG. S3 Funnel plot for publication bias test of perioperative findings. A ERCP-related adverse effects. B Blood loss. C Operation time. D Surgical complications (P = 0.34, Harbord test). E Mortality. ERCP, endoscopic retrograde cholangiopancreatography (DOCX 422 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Endo, Y., Tanaka, M., Kitago, M. et al. Comparison Between Plastic and Metallic Biliary Stent Placement for Preoperative Patients with Pancreatic Head Cancer: A Systematic Review and Meta-Analysis. Ann Surg Oncol 31, 1319–1327 (2024). https://doi.org/10.1245/s10434-023-14523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14523-y