Abstract
Objective
We aim to delineate the relationship between breast and axillary pathologic complete response (pCR) in patients receiving neoadjuvant chemotherapy for breast cancer.
Methods
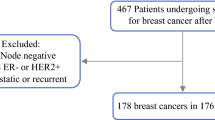
We performed a retrospective cohort study of patients with clinical T1–4N0–3M0 breast cancer receiving neoadjuvant chemotherapy followed by surgical therapy at Sunnybrook Health Sciences Centre in Toronto, Canada between 2014 and 2019. Clinicopathologic data were abstracted from the electronic medical record. Women were stratified into receptor subtypes as follows: hormone receptor positive (HR+)/human epidermal growth factor receptor 2 negative (HER2–), HR+/HER2+, HR–/HER2+ and HR–/HER2– (triple negative) and compared with Fisher’s exact test. Our primary outcome was to assess the positive predictive value of breast pCR for determining axillary pCR, and vice versa.
Results
There were 374 breast cancers, with 109 (29.1%) achieving breast pCR (ypT0/Tis). Amongst node-positive tumours achieving breast pCR, rates of associated axillary pCR (ypN0/0i+) were as follows: HR+/HER2– (2/6, 33.3%), HR+/HER2+ (12/13, 92.3%), HR–/HER2+ (15/17, 88.2%) and triple negative (15/17, 88.2%) (P = 0.02). Conversely, amongst node-positive tumours achieving axillary pCR, rates of associated breast pCR were: HR+/HER2– (2/10, 20.0%), HR+/HER2+ (12/23, 52.2%), HR–/HER2+ (15/24, 62.5%) and triple negative (15/26, 57.7%) (P = 0.1).
Conclusions
Breast pCR is a strong predictor of axillary pCR in women with HER2-positive and triple-negative breast cancers. Conversely, axillary pCR is a modest predictor of breast pCR for these subtypes. There is a poor relationship between breast and axillary pCR in women with hormone receptor-positive disease. These data may inform future de-escalation of surgery in women with HER2-positive and triple-negative disease.


Similar content being viewed by others
References
Cortazar P, Zhang L, Untch M, et al. Pathologic complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
Kuerer HM, Newman LA, Buzdar AU, et al. Pathologic tumor response in the breast following neoadjuvant chemotherapy predicts axillary lymph node status. Cancer J Sci Am. 1998;4(4):230–6.
Leon-Ferre RA, Hieken TJ, Boughey JC. The landmark series: neoadjuvant chemotherapy for triple-negative and HER2-positive breast cancer. Ann Surg Oncol. 2021;28(4):2111–9.
Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59.
von Minckwitz G, Huang CS, Mano MS, et al. KATHERINE Investigators. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28.
Heil J, Pfob A, Sinn HP, et al. RESPONDER Investigators. Diagnosing pathologic complete response in the breast after neoadjuvant systemic treatment of breast cancer patients by minimal invasive biopsy: oral presentation at the San Antonio Breast Cancer Symposium on Friday, December 13, 2019. Ann Surg. 2020. https://doi.org/10.1097/SLA.0000000000004246.
Tasoulis MK, Lee HB, Yang W, et al. Accuracy of post-neoadjuvant chemotherapy image-guided breast biopsy to predict residual cancer. JAMA Surg. 2020; 155(12): e204103.
Basik M, Cecchini RS, De Los Santos JF, et al. Abstract GS5-05: Primary analysis of NRG-BR005, a phase II trial assessing accuracy of tumour bed biopsies in predicting pathologic complete response (pCR) in patients with clinical/radiological complete response after neoadjuvant chemotherapy (NCT) to explore the feasibility of breast-conserving treatment without surgery. Cancer Res. 2020. 80(4 Suppl): Abstract #GS5-05.
van Loevezijn AA, van der Noordaa MEM, van Werkhoven ED, et al. Minimally invasive complete response assessment of the breast after neoadjuvant systemic therapy for early breast cancer (MICRA trial): interim analysis of a multicentre observational cohort study. Ann Surg Oncol. 2021;28(6);3243–53.
Sutton EJ, Braunstein LZ, El-Tamer M, et al. Accuracy of magnetic resonance imaging-guided biopsy to verify breast cancer pathologic complete response after neoadjuvant chemotherapy: a nonrandomized controlled trial. JAMA Netw Open. 2021; 4(4): e2034045.
Lee HB, Han W, Kim SY, et al. Prediction of pathologic complete response using image-guided biopsy after neoadjuvant chemotherapy in breast cancer patients selected based on MRI findings: a prospective feasibility trial. Breast Cancer Res Treat. 2020;182(1):97–105.
Barron AU, Hoskin TL, Day CN, Hwang ES, Kuerer HM, Boughey JC. Association of low nodal positivity rate among patients with ERBB2-positive or triple-negative breast cancer and breast pathologic complete response to neoadjuvant chemotherapy. JAMA Surg. 2018;153(12):1120–6.
Vugts G, Van den Heuvel F, Maaskant-Braat AJG, et al. Predicting breast and axillary response after neoadjuvant treatment for breast cancer: the role of histology vs receptor status. Breast J. 2018;24(6):894–901.
Samiei S, van Nijnatten TJA, de Munck L, et al. Correlation between pathologic complete response in the breast and absence of axillary lymph node metastases after neoadjuvant systemic therapy. Ann Surg. 2020;271(3):574–80.
Hong J, Tong Y, He J, Chen X, Shen K. Association between tumor molecular subtype, clinical stage and axillary pathologic response in breast cancer patients undergoing complete pathologic remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery. Ther Adv Med Oncol. 2021;13:1758835921996673.
Schmidt H, Zhaveri S, Valente C, et al. Response in the breast vs axilla after neoadjuvant treatment and implications for nonoperative management of invasive breast cancer. Breast J. 2021;27(2):120–5.
Wang Y, Li L, Liu X, et al. Treatment response correlation between primary tumor and axillary lymph nodes after neoadjuvant therapy in breast cancer: a retrospective study based on real-world data. Gland Surg. 2021;10(2):656–69.
Shi ZQ, Qui PF, Liu YB, et al. Neo-adjuvant chemotherapy and axillary de-escalation management for patients with clinically node-negative breast cancer. Breast J. 2019;25(6):1154–9.
Choi HJ, Ryu JM, Kim I, et al. Prediction of axillary pathologic response with breast pathologic complete response after neoadjuvant chemotherapy. Breast Cancer Res Treat. 2019;176(3):591–6.
Tadros AB, Yang WT, Krishnamurthy S, et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg. 2017;152(7):665–70.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007; 370(9596): 1453-7.
AJCC (American Joint Committee on Cancer) Cancer Staging Manual; 8th Edition, 3rd printing, Amin MB, Edge SB, Greene FL, et al (Eds), Springer, Chicago 2018.
Allison KH, Hammon MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. J Clin Oncol. 2020;38(12):1346–66.
Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(20):2105–22.
U.S. Department of Health and Human Services, Food and Drug Administration, Oncology Center of Excellence, Centre for Drug Evaluation and Research, Center for Biologics Evaluation and Research. (July 2020). Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval Guidance for Industry. https://www.fda.gov/media/83507/download [Accessed 13 June 2021].
Comparison of axillary lymph node dissection with axillary radiation for patients with node-positive breast cancer treated with chemotherapy. ClinicalTrials.gov identifier: NCT01901094. Updated March 8, 2021. Accessed April 8, 2021. https://www.clinicaltrials.gov/ct2/show/NCT01901094
Mamounas EP, Bandos H, White JR, et al. NRG Oncology/NSABP B-51/RTOG 1304: phase III trial to determine if chest wall and regional nodal radiotherapy (CWRNRT) post mastectomy (Mx) or the addition of RNRT to whole breast RT post breast-conserving surgery (BCS) reduces invasive breast cancer recurrence-free interval (IBCR-FI) in patients (pts) with pathologically positive axillary (PPAx) nodes who are ypN0 after neoadjuvant chemotherapy (NC). J Clin Oncol. 2019; 37(suppl 15). doi: https://doi.org/10.1200/JCO.2019/37.15_suppl.TPS600
Racz JM, Caudle AS. Sentinel node lymph node surgery after neoadjuvant therapy: principles and techniques. Ann Surg Oncol. 2019;26(10):3040–5.
Samiei S, Simons JM, Engelen SME, Beets-Tan RGH, Classe JM, Smidt ML; EUBREAST Group. Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: a systematic review and meta-analysis. JAMA Surg. 2021; 156(6): e210891. doi: https://doi.org/10.1001/jamasurg.2021.0891.
Boughey JC, Ballman KV, McCall LM, et al. Tumour biology and response to chemotherapy impact breast cancer-specific survival in node-positive breast cancer patients treated with neoadjuvant chemotherapy: long-term follow-up from ACOSOG Z1071 (Alliance). Ann Surg. 2017;266(4):667–76.
von Minckwitz G, Untch M, Blohmer J, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804.
Pardo JA, Fan B, Mele A, et al. The role of Oncotype DX® recurrence score in predicting axillary response after neoadjuvant chemotherapy in breast cancer. Ann Surg Oncol. 2021;28(3):1320–5.
Pease AM, Riba LA, Gruner RA, Tung NM, James TA. Oncotype DX® recurrence score as a predictor of response to neoadjuvant chemotherapy. Ann Surg Oncol. 2019;26(2):366–71.
Boughey JC, Suman VJ, Mittendorf EA, et al. Alliance for clinical trials in oncology sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–61.
Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–18.
Goel N, Yadegarynia S, Rodgers S, et al. Axillary response rates to neoadjuvant chemotherapy in breast cancer patients with advanced nodal disease. J Surg Oncol. 2021;124(1):25–32.
Garcia-Tejedor A, Fernandez-Gonzalez S, Ortega R, et al. Can we avoid axillary lymph node dissection in N2 breast cancer patients with chemo-sensitive tumours such as HER2 and TNBC? Breast Cancer Res Treat. 2021;185(3):657–66.
Acknowledgements
D.W.L. is supported by the Canadian Institutes of Health Research (CIHR) Fellowship from 2019 to 2022. He is also supported by the Helen Marion Walker-Soroptimist Women’s Health Research Scholarship and the Canadian Cancer Society Chair in Breast Cancer Research at Women’s College Research Institute (Women’s College Hospital, Toronto, Ontario, Canada).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
D.W.L. and B.D.G. have no conflicts of inflicts. N.J.L.H. is a consultant for MOLLI Surgical.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was presented as a Quickshot oral presentation on 1 May 2021 at the 22nd Annual Meeting of the American Society of Breast Surgeons (ASBrS) and received the 2021 ASBrS Scientific Impact Recognition Award for best presentation as voted on by the audience at the ASBrS Annual Meeting.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, D.W., Greene, B.D. & Look Hong, N.J. Relationship Between Breast and Axillary Pathologic Complete Response in Women Receiving Neoadjuvant Chemotherapy for Breast Cancer. Ann Surg Oncol 28, 5495–5506 (2021). https://doi.org/10.1245/s10434-021-10519-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10519-8