Abstract
Background
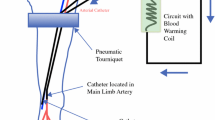
Isolated limb infusion (ILI) is a minimally invasive technique delivering regional chemotherapy to treat in-transit extremity melanoma. Determining perioperative factors that could predict toxicity is important to optimize strategies to improve clinical outcomes after regional chemotherapy in melanoma.
Methods
Perioperative factors from 171 ILI patients performed at eight centers from 2001 to 2008 were reviewed. The Wieberdink limb toxicity scale and creatine phosphokinase (CK) levels were used to measure toxicity. Logistic regression analysis was used to estimate the association between toxicity and perioperative parameters.
Results
Mild (grades I–II) and severe (grades ≥III) limb toxicity developed in 68% and 32% of patients, respectively. Melphalan adjusted for ideal body weight (aIBW) and papaverine were used in 47% and 63% of patients, respectively. Median peak CK for all patients was 563 U/l, and median peak occurred at postoperative day 4. On univariate analysis, papaverine and high CK levels (>563 U/l) were significantly associated with higher toxicity. On the contrary, aIBW was significantly associated with a lower risk of severe toxicity. Perfusate blood gas at 30 min [pH, PaO2, and base excess (BE) ], limb temperature, and ischemia time were not predictive of limb toxicity. On multivariate analysis, severe toxicity was associated with female sex (P = 0.01), papaverine (P = 0.01), and high peak CK levels (P < 0.01). Independent predictors of high CK levels included younger age, unadjusted melphalan dose, and low PaO2 at 30 min.
Conclusions
ILI can be performed with an acceptable morbidity. Papaverine use, female gender, and high peak CK were associated with higher limb toxicity. CK levels can be diminished significantly with aIBW.


Similar content being viewed by others
References
Borgstein PJ, Meijer S, van Diest PJ. Are locoregional cutaneous metastases in melanoma predictable? Ann Surg Oncol. 1999;6(3):315–21.
Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of intransit melanoma after sentinel lymphadenectomy. Ann Surg Oncol. 2005;12(8):587–96.
Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635–48.
Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54(3):131–49; quiz 182–34.
Gimbel MI, Delman KA, Zager JS. Therapy for unresectable recurrent and intransit extremity melanoma. Cancer Control. 2008;15(3):225–32.
Creech O Jr, Krementz ET, Ryan RF, Winblad JN. Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg. 1958;148(4):616–32.
Minor DR, Allen RE, Alberts D, et al. A clinical and pharmacokinetic study of isolated limb perfusion with heat and melphalan for melanoma. Cancer. 1985;55(11):2638–44.
Di Filippo F, Calabro A, Giannarelli D, et al. Prognostic variables in recurrent limb melanoma treated with hyperthermic antiblastic perfusion. Cancer. 1989;63(12):2551–61.
Klaase JM, Kroon BB, van Geel AN, et al. Prognostic factors for tumor response and limb recurrence-free interval in patients with advanced melanoma of the limbs treated with regional isolated perfusion with melphalan. Surgery. 1994;115(1):39–45.
Thompson JF, Hunt JA, Shannon KF, Kam PC. Frequency and duration of remission after isolated limb perfusion for melanoma. Arch Surg. 1997;132(8):903–7.
Cornett WR, McCall LM, Petersen RP, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol. 2006;24(25):4196–201.
Moller MG, Lewis JM, Dessureault S, Zager JS. Toxicities associated with hyperthermic isolated limb perfusion and isolated limb infusion in the treatment of melanoma and sarcoma. Int J Hyperthermia. 2008;24(3):275–89.
Lens MB, Dawes M. Isolated limb perfusion with melphalan in the treatment of malignant melanoma of the extremities: a systematic review of randomised controlled trials. Lancet Oncol. 2003;4(6):359–64.
Hoven-Gondrie ML, Thijssens KM, Van den Dungen JJ, et al. Long-term locoregional vascular morbidity after isolated limb perfusion and external-beam radiotherapy for soft tissue sarcoma of the extremity. Ann Surg Oncol. 2007;14(7):2105–12.
Eggimann P, Chiolero R, Chassot PG, et al. Systemic and hemodynamic effects of recombinant tumor necrosis factor alpha in isolation perfusion of the limbs. Chest. 1995;107(4):1074–82.
Grunhagen DJ, van Etten B, Brunstein F, et al. Efficacy of repeat isolated limb perfusions with tumor necrosis factor alpha and melphalan for multiple in-transit metastases in patients with prior isolated limb perfusion failure. Ann Surg Oncol. 2005;12(8):609–15.
Vrouenraets BC, Keus RB, Nieweg OE, Kroon BB. Complications of combined radiotherapy and isolated limb perfusion with tumor necrosis factor alpha ± interferon gamma and melphalan in patients with irresectable soft tissue tumors. J Surg Oncol. 1997;65(2):88–94.
Thompson JF, Waugh RC, Saw RPM, Kam PCA. Isolated limb infusion with melphalan for recurrent limb melanoma: a simple alternative to isolated limb perfusion. Reg Cancer Treat. 1994;7:188–92.
Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol. 1998;14(3):238–47.
Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes Following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol. 2008;15(11):3003–13.
Zager JS, Gershenwald JE, Aldrink EL, et al. Isolated limb infusion for locally recurrent and in-transit extremity melanoma: a combined institutional initial experience. Ann Surg Oncol. 2006;13(2):s84.
Brady MS, Brown K, Patel A, Fisher C, Marx W. A phase II trial of isolated limb infusion with melphalan and dactinomycin for regional melanoma and soft tissue sarcoma of the extremity. Ann Surg Oncol. 2006;13(8):1123–9.
Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15(8):2195–205.
Beasley GM, Caudle A, Petersen R, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the United States. J Am Coll Surg. 2009;In press.
Lindner P, Doubrovsky A, Kam PC, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9(2):127–36.
Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18(10):905–10.
Klaase JM, Kroon BB, van Geel BN, et al. Patient- and treatment-related factors associated with acute regional toxicity after isolated perfusion for melanoma of the extremities. Am J Surg. 1994;167(6):618–20.
Vrouenraets BC, Hart GA, Eggermont AM, et al. Relation between limb toxicity and treatment outcomes after isolated limb perfusion for recurrent melanoma. J Am Coll Surg. 1999;188(5):522–30.
Thompson JF, Lai DT, Ingvar C, Kam PC. Maximizing efficacy and minimizing toxicity in isolated limb perfusion for melanoma. Melanoma Res. 1994;4(Suppl 1):45–50.
Bonenkamp JJ, Thompson JF, de Wilt JH, et al. Isolated limb infusion with fotemustine after dacarbazine chemosensitisation for inoperable loco-regional melanoma recurrence. Eur J Surg Oncol. 2004;30(10):1107–12.
Vrouenraets BC, Eggermont AM, Hart AA, et al. Regional toxicity after isolated limb perfusion with melphalan and tumour necrosis factor-alpha versus toxicity after melphalan alone. Eur J Surg Oncol. 2001;27(4):390–5.
Vrouenraets BC, Klaase JM, Kroon BB, et al. Long-term morbidity after regional isolated perfusion with melphalan for melanoma of the limbs. The influence of acute regional toxic reactions. Arch Surg. 1995;130(1):43–7.
Klaase JM, Kroon BB, Beijnen JH, van Slooten GW, van Dongen JA. Melphalan tissue concentrations in patients treated with regional isolated perfusion for melanoma of the lower limb. Br J Cancer. 1994;70(1):151–3.
Edwards MJ, Soong SJ, Boddie AW, Balch CM, McBride CM. Isolated limb perfusion for localized melanoma of the extremity. A matched comparison of wide local excision with isolated limb perfusion and wide local excision alone. Arch Surg. 1990;125(3):317–21.
Parsons PG, Carter FB, Morrison L, Regius Mary S. Mechanism of melphalan resistance developed in vitro in human melanoma cells. Cancer Res. 1981;41(4):1525–34.
Krementz ET, Carter RD, Sutherland CM, et al. Regional chemotherapy for melanoma. A 35-year experience. Ann Surg. 1994;220(4):520–34; discussion 534–25.
Vrouenraets BC, Kroon BB, Klaase JM, et al. Value of laboratory tests in monitoring acute regional toxicity after isolated limb perfusion. Ann Surg Oncol. 1997;4(1):88–94.
Hohenberger P, Haier J, Schlag PM. Rhabdomyolysis and renal function impairment after isolated limb perfusion–comparison between the effects of perfusion with rhTNF alpha and a ‘triple-drug’ regimen. Eur J Cancer. 1997;33(4):596–601.
Cheng TY, Grubbs E, Abdul-Wahab O, et al. Marked variability of melphalan plasma drug levels during regional hyperthermic isolated limb perfusion. Am J Surg. 2003;186(5):460–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santillan, A.A., Delman, K.A., Beasley, G.M. et al. Predictive Factors of Regional Toxicity and Serum Creatine Phosphokinase Levels After Isolated Limb Infusion for Melanoma: A Multi-Institutional Analysis. Ann Surg Oncol 16, 2570–2578 (2009). https://doi.org/10.1245/s10434-009-0563-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0563-9