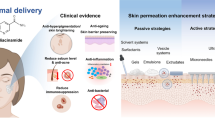
Abstract
Oral candidiasis is a fungal infection affecting the oral mucous membrane, and this research specifically addresses on a localized treatment through fluconazole-loaded ibuprofen in situ gel-based oral spray. The low solubility of ibuprofen is advantageous for forming a gel when exposed to an aqueous phase. The 1% w/w fluconazole-loaded in situ gel oral sprays were developed utilizing various concentrations of ibuprofen in N-methyl pyrrolidone. The prepared solutions underwent evaluation for viscosity, surface tension, contact angle, water tolerance, gel formation, interface interaction, drug permeation, and antimicrobial studies. The higher amount of ibuprofen reduced the surface tension and retarded solvent exchange. The use of 50% ibuprofen as a gelling agent demonstrated prolonged drug permeation for up to 24 h. The incorporation of Cremophor EL in the formulations resulted in increased drug permeation and exhibited effective inhibition against Candida albicans, Candida krusei, Candida lusitaniae, and Candida tropicalis. While the Cremophor EL-loaded formulation did not exhibit enhanced antifungal effects on agar media, its ability to facilitate the permeation of fluconazole and ibuprofen suggested potential efficacy in countering Candida invasion in the oral mucosa. Moreover, these formulations demonstrated significant thermal inhibition of protein denaturation in egg albumin, indicating anti-inflammatory properties. Consequently, the fluconazole-loaded ibuprofen in situ gel-based oral spray presents itself as a promising dosage form for oropharyngeal candidiasis treatment.
Graphical Abstract











Similar content being viewed by others
Data Availability
The data presented in this study are available on the request from the corresponding author.
References
Epstein JB, Polsky B. Oropharyngeal candidiasis: a review of its clinical spectrum and current therapies. Clin Ther. 1998;20:40–57. https://doi.org/10.1016/s0149-2918(98)80033-7.
Sivannana S, Vishnuvardhana A, Elumalaib K, Srinivasanc S, Cherianb BV, Ramanujamd SK, et al. Azithromycin and co-trimoxazole-induced oral thrush: a case report from the perspective of pharmacy. Intell Pharm. 2023;1(4):280–2. https://doi.org/10.1016/j.ipha.2023.06.007.
Pisano M, Romano A, Di Palo MP, Baroni A, Serpico R, Contaldo M. Oral candidiasis in adult and pediatric patients with COVID-19. Biomedicines. 2023;11:846. https://doi.org/10.3390/biomedicines11030846.
Karajacob AS, Azizan NB, Al-Maleki AR, Goh JP, Loke MF, Khor HM, et al. Candida species and oral mycobiota of patients clinically diagnosed with oral thrush. PLoS ONE. 2023;18(4):e0284043. https://doi.org/10.1371/journal.pone.0284043.
Fang J, Huang B, Ding Z. Efficacy of antifungal drugs in the treatment of oral candidiasis: a Bayesian network meta-analysis. J Prosthet Dent. 2021;125(2):257–65. https://doi.org/10.1016/j.prosdent.2019.12.025.
Lucatorto FM, Franker C, Hardy D, Chafey S, Calif LA. Treatment of refractory oral candidiasis with fluconazole. Oral Surg Oral Med Oral Pathol. 1991;71:42–4. https://doi.org/10.1016/0030-4220(91)90518-h.
Elias R, Basu P, Fridman M. Fluconazole-COX inhibitor hybrids: a dual-acting class of antifungal azoles. J Med Chem. 2022;65:2361–73. https://doi.org/10.1021/acs.jmedchem.1c01807.
Yücesoy M, Öktem IMA, Gülay Z. In-vitro synergistic effect of fluconazole with nonsteroidal anti-inflammatory agents against Candida albicans strains. J Chemother. 2000;12(5):385–9. https://doi.org/10.1179/joc.2000.12.5.385.
Scott EM, Tariq VN, Mccrory RM. Demonstration of synergy with fluconazole and either ibuprofen, sodium salicylate, or propylparaben against Candida albicans In Vitro. Antimicrob Agents Chemother. 1995;39(12):261–2614. https://doi.org/10.1128/AAC.39.12.2610.
Costa-de-Oliveira S, Miranda IM, Silva-Dias A, Silva AP, Rodrigues AG, Pina-Vaz C. Ibuprofen potentiates the in vivo antifungal activity of fluconazole against Candida albicans murine infection. Antimicrob Agents Chemother. 2015;59:4289–92. https://doi.org/10.1128/AAC.05056-14.
Mohamed SP, Muzzammil S, Pramod KT. Preparation of fluconazole buccal tablet and influence of formulation expedients on its properties. Yao Xue Xue Bao. 2011;46(4):460–5.
Tejada G, Calvo NL, Morri M, Sortino M, Lamas C, Álvarez VA, Leonardi D. Miconazole nitrate microparticles in lidocaine loaded films as a treatment for oropharyngeal candidiasis. Materials. 2023;16:3586. https://doi.org/10.3390/ma16093586.
Ardizzoni A, Boaretto G, Pericolini E, Pinetti D, de Capezzone JA, Durando L, et al. Effects of benzydamine and mouthwashes containing benzydamine on Candida albicans adhesion, biofilm formation, regrowth, and persistence. Clin Oral Invest. 2022;26:3613–25. https://doi.org/10.1007/s00784-021-04330-8.
Kanagalingam J, Feliciano R, Hah JH, Labib H, Le TA, Lin JC. Practical use of povidone-iodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infections. Int J Clin Pract. 2015;69(11):1247–56. https://doi.org/10.1111/ijcp.12707.
Nittayananta W, Limsuwan S, Srichana T, Sae-Wong C, Amnuaikit T. Oral spray containing plant-derived compounds is effective against common oral pathogens. Arch Oral Biol. 2018;90:80–5. https://doi.org/10.1016/j.archoralbio.2018.03.002.
Jitrangsri K, Lertsuphotvanit N, Kabthong N, Phaechamud T. Metronidazole loaded camphor based in situ forming matrix for periodontitis treatment. AAPS PharmSciTech. 2023;24:185. https://doi.org/10.1208/s12249-023-02640-6.
Puyathorn N, Lertsuphotvanit N, Chantadee T, Pichayakorn W, Phaechamud T. Lincomycin HCl-loaded borneol-based in situ gel for periodontitis treatment. Gels. 2023;9:495. https://doi.org/10.3390/gels9060495.
Puyathorn N, Senarat S, Lertsuphotvanit N, Phaechamud T. Physicochemical and bioactivity characteristics of doxycycline hyclate-loaded solvent removal-induced ibuprofen-based in situ forming gel. Gels. 2023;9:128. https://doi.org/10.3390/gels9020128.
Lizambard M, Menu T, Fossart M, Bassand C, Agossa K, Huck O, et al. In-situ forming implants for the treatment of periodontal diseases: simultaneous controlled release of an antiseptic and an anti-inflammatory drug. Int J Pharm. 2019;15:118833. https://doi.org/10.1016/j.ijpharm.2019.118833.
Li X, Fan R, Wang Y, Wu M, Tong A, Shi J, et al. In situ gel-forming dual drug delivery system for synergistic combination therapy of colorectal peritoneal carcinomatosis. RSC Adv. 2015;5:101494–506.
Batool F, Agossa K, Lizambard M, Petit C, Bugueno IM, Delcourt-Debruyne E, et al. In-situ forming implants loaded with chlorhexidine and ibuprofen for periodontal treat-ment: proof of concept study in vivo. Int J Pharm. 2019;5:118564. https://doi.org/10.1016/j.ijpharm.2019.118564.
Puyathorn N, Sirirak J, Chantadee T, Phaechamud T. Phase separation and intermolecular binding energy of ibuprofen in some organic solvents. Mater Today: Proc. 2022;65(4):2303–8. https://doi.org/10.1016/j.matpr.2022.05.030.
Bello OS, Alagbada TC, Alao OC, Olatunde AM. Sequestering a non-steroidal anti-inflammatory drug using modified orange peels. Appl Water Sci. 2020;10:172. https://doi.org/10.1007/s13201-020-01254-8.
** J, Chang Q, Chan CK, Meng ZY, Wang GN, Sun JB, Wang YT, Tong HH, Zheng Y. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS PharmSciTech. 2009;10(1):172–82. https://doi.org/10.1208/s12249-009-9190-9.
Srichan T, Phaechamud T. Designing solvent exchange-induced in situ forming gel from aqueous insoluble polymers as matrix base for periodontitis treatment. AAPS PharmSciTech. 2017;18(1):194–201. https://doi.org/10.1208/s12249-016-0507-1.
Ike E, Ezike SC. Estimation of viscosity Arrhenius pre-exponential factor and activation energy of some organic liquids. Int J Recent Res Phys Chem Sci. 2018;5(1):18–26.
Hsin WL, Sheng YJ, Lin SY, Tsao HK. Surface tension increment due to solute addition. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;69:031605. https://doi.org/10.1103/PhysRevE.69.031605.
Golmaghani-Ebrahimi E, Bagheri A, Fazli M. The influence of temperature on surface concentration and interaction energy between components in binary liquid systems. J Chem Thermodyn. 2020;146:10615. https://doi.org/10.1016/j.jct.2020.106105.
Qazi MJ, Schlegel SJ, Backus EGH, Bonn M, Bonn D, Shahidzadeh N. Dynamic surface tension of surfactants in the presence of high salt concentrations. Langmuir. 2020;36:7956–64. https://doi.org/10.1021/acs.langmuir.0c01211.
Lertsuphotvanit N, Sirirak J, Tamdee P, Tuntarawongsa S, Phaechamud T, Chantadee T. Ways to assess and regulate the performance of a bi-mechanism-induced borneol-based in situ forming matrix. Pharmaceutics. 2023;15:2053. https://doi.org/10.3390/pharmaceutics15082053.
Khaing EM, Intaraphairot T, Santimaleeworagun W, Phorom Y, Chuenbarn T, Phaechamud T. Natural-resin in-situ-forming gels: physicochemical characteristics and bioactivities. Pharm Sci Asia. 2021;48:461–70. https://doi.org/10.29090/psa.2021.05.20.077.
Senarat S, Charoenteeraboon J, Praphanwittaya P, Phaechamud T. Phase behavior of doxycycline hyclate-incorporated bleached shellac in-situ forming gel/microparticle after solvent movement. Key Eng Mater. 2020;859:21–6. https://doi.org/10.4028/www.scientific.net/KEM.859.21.
Lertsuphotvanit N, Tuntarawongsa S, Jitrangsri K, Phaechamud T. Clotrimazole-loaded borneol-based in situ forming gel as oral sprays for oropharyngeal candidiasis therapy. Gels. 2023;9:412. https://doi.org/10.3390/gels9050412.
Chantadee T, Santimaleeworagun W, Phorom Y, Chuenbarn T, Phaechamud T. Saturated fatty acid-based in situ forming matrices for localized antimicrobial delivery. Pharmaceutics. 2020;12:808. https://doi.org/10.3390/pharmaceutics12090808.
Himawan C, Starov VM, Stapley AG. Thermodynamic and kinetic aspects of fat crystallization. Adv Colloid Interface Sci. 2006;122:3–33. https://doi.org/10.1016/j.cis.2006.06.016.
Rahman M, Ahmad S, Tarabokija J, Bilgili E. Roles of surfactant and polymer in drug release from spray-dried hybrid nanocrystal-amorphous solid dispersions (HyNASDs). Powder Technol. 2020;361:663–78. https://doi.org/10.1016/j.powtec.2019.11.058.
Do MP, Neut C, Delcourt E, Certo ST, Siepmann J, Siepmann F. In situ forming implants for periodontitis treatment with improved adhesive properties. Eur J Pharm Biopharm. 2014;88(2):342–50. https://doi.org/10.1016/j.ejpb.2014.05.006.171.
Puyathorn N, Tamdee P, Sirirak J, Okonogi S, Phaechamud T, Chantadee T. Computational insight of phase transformation and drug release behaviour of doxycycline-loaded ibuprofen-based in-situ forming gel. Pharmaceutics. 2023;15:2315. https://doi.org/10.3390/pharmaceutics15092315.
Lipson BK, Yannuzzi LA. Complications of intravenous fluorescein injections. Int Ophthalmol Clin. 1989;29:200–5. https://doi.org/10.1097/00004397-198902930-00011.
Alemán-Nava GS, Cuellar-Bermudez SP, Cuaresma M, Bosma R, Muylaert K, Ritmann BE, et al. How to use Nile red, a selective fluorescent stain for microalgal neutral lipids. J Microbiol Methods. 2016;128:74–9.
Martinez V, Henary M. Nile red and Nile blue: applications and syntheses of structural analogues. Chemistry. 2016;22(39):13764–82.
Senarat S, Tuntarawongsa S, Lertsuphotvanit N, Rojviriya C, Phaechamud T, Chantadee T. Levofloxacin HCl-loaded eudragit L-based solvent exchange-induced in situ forming gel using monopropylene glycol as a solvent for periodontitis treatment. Gels. 2023;9:583. https://doi.org/10.3390/gels9070583.
El-Housiny S, Shams Eldeen MA, El-Attar YA, Salem HA, Attia D, Bendas ER, et al. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: formulation and clinical study. Drug Deliv. 2018;25(1):78–90. https://doi.org/10.1080/10717544.2017.1413444.
Jacob S, Nair AB, Boddu SHS, Gorain B, Sreeharsha N, Shah J. An updated overview of the emerging role of patch and film-based buccal delivery systems. Pharmaceutics. 2021;13:1206. https://doi.org/10.3390/pharmaceutics13081206.
Yehia SA, El-Gazayerly ON, Basalious EB. Fluconazole mucoadhesive buccal films: in vitro/in vivo performance. Curr Drug Deliv. 2009;6(1):17–27. https://doi.org/10.2174/156720109787048195.
Hmingthansanga V, Singh N, Banerjee S, Manickam S, Velayutham R, Natesan S. Improved topical drug delivery: role of permeation enhancers and advanced approaches. Pharmaceutics. 2022;14:2818. https://doi.org/10.3390/pharmaceutics14122818.
Darío A, Tinjacá FM, Almanza OA, Jouyban A, Acree WE Jr. Effect of N-methyl-pyrrolidone (NMP) on the equilibrium solubility of meloxicam in aqueous media: correlation, dissolution thermodynamics, and preferential solvation. ACS Omega. 2022;7:37988–8002. https://doi.org/10.1021/acsomega.2c05189.
Varrassi G, Pergolizzi JV, Dowling P, Paladini A. Ibuprofen safety at the golden anniversary: are all NSAIDs the same? A narrative review. Adv Ther. 2020;37:61–82. https://doi.org/10.1007/s12325-019-01144-9.
Mazaleuskaya LL, Theken KN, Gong L, Thorn CF, FitzGerald GA, Altman RB, et al. Pharm GKB summary: ibuprofen pathways. Pharmacogenet Genom. 2015;25(2):96–106. https://doi.org/10.1097/FPC.0000000000000113.
Villanueva M, Heckenberger R, Strobach H, Palmér M, Schrör K. Equipotent inhibition by R(−)-, S(+)- and racemic ibuprofen of human polymorphonuclear cell function in vitro. Br J Clin Pharmacol. 1993;35:235–42.
Abuhajar E, Ali K, Zulfiqar G, Al Ansari K, Raja HZ, Bishti S, et al. Management of chronic atrophic candidiasis (denture stomatitis)-a narrative review. Int J Environ Res Public Health. 2023;20(4):3029. https://doi.org/10.3390/ijerph20043029.
Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun. 2007;75:2126–35. https://doi.org/10.1128/IAI.00054-07.
Swidergall M, Filler SG. Oropharyngeal candidiasis: fungal invasion and epithelial cell responses. PLoS Pathog. 2017;13(1):e1006056. https://doi.org/10.1371/journal.ppat.1006056.
Phaechamud T, Mahadlek J, Charoenteeraboon J, Choopun S. Characterization and antimicrobial activity of N-methyl-2-pyrrolidone-loaded ethylene oxide-propylene oxide block copolymer thermosensitive gel. Indian J Pharm Sci. 2012;74:498. https://doi.org/10.4103/0250-474x.110574.
Iamir EM, Ibrahim SY. Physical properties of aqueous N-methyl pyrrolidone at different temperatures. Pet Sci Technol. 2004;22:1571–9. https://doi.org/10.1081/LFT-200027883.
Büchter A, Meyer U, Kruse-Lösler B, Joos U, Kleinheinz J. Sustained release of doxycycline for the treatment of peri-implantitis: randomised controlled trial. Br J Oral Maxillofac Surg. 2004;42:439–44. https://doi.org/10.1016/j.bjoms.2004.06.005.
Zeng L, **n X, Zhang Y. Development and characterization of promising Cremophor EL-stabilized o/w nanoemulsions containing short-chain alcohols as a cosurfactant. RSC Adv. 2017;7:19815–27. https://doi.org/10.1039/C6RA27096D.
Scripture CD, Figg WD, Sparreboom A. Paclitaxel chemotherapy: from empiricism to a mechanism-based formulation strategy. Ther Clin Risk Manag. 2005;1(2):107–14. https://doi.org/10.2147/tcrm.1.2.107.62910.
Gullo FP, Rossi SA, Sardi J, Teodoro VLI, Mendes-Giannini MJS, Fusco-Almeida AM. Cryptococcosis: epidemiology, fungal resistance, and new alternatives for treatment. Eur J Clin Microbiol Infect Dis. 2013;32(11):1377–91.
Rocha LF, Pippi B, Fuentefria AM, Mezzari A. Synergistic effect of ibuprofen with itraconazole and fluconazole against Cryptococcus neoforman. Braz J Pharm Sci. 2020;56:e18599. https://doi.org/10.1590/s2175-97902019000318599.
Argenta JS, Alves SH, Silveira F, Maboni G, Zanette RA, Cavalheiro AS, et al. In vitro and in vivo susceptibility of two-drug and three-drug combinations of terbinafine, itraconazole, caspofungin, ibuprofen and fluvastatin against Pythium insidiosum. Vet Microbiol. 2012;157(1–2):137–42.
Rusu E, Radu-Popescu M, Pelinescu D, Vassu T. Treatment with some anti-inflammatory drugs reduces germ tube formation in Candida albicans strains. Braz J Microbiol. 2014;45(4):1379–83.
Leelaprakash G, Dass SM. In vitro anti-inflammatory activity of methanol extract of Enicostemma axillare. Int J Drug Dev Res. 2011;3:189–96.
Kumarasinghe N, Dharmadeva S, Galgamuwa L, Prasadinie C. In vitro anti-inflammatory activity of Ficus racemosa L. bark using albumin denaturation method. Int Q J Res Ayurveda. 2018;39:239. https://doi.org/10.4103/ayu.ayu_27_18.
Oesterreicher Z, Eberl S, Zeitlinger M. Impact of different antimycotics on cytokine levels in an in vitro aspergillosis model in human whole blood. Infection. 2020;48:65–73. https://doi.org/10.1007/s15010-019-01346-x.
Chaiya P, Senarat S, Phaechamud T, Narakornwit W. In vitro anti-inflammatory activity using thermally inhibiting protein denaturation of egg albumin and antimicrobial activities of some organic solvents. Mater Today: Proc. 2022;65:2290–5. https://doi.org/10.1016/j.matpr.2022.04.916.
Roche-Molina M, Hardwick B, Sanchez-Ramos C, Sanz-Rosa D, Gewert D, Cruz FM, et al. The pharmaceutical solvent N-methyl-2-pyrrolidone (NMP) attenuates inflammation through Krüppel-like factor 2 activation to reduce atherogenesis. Sci Rep. 2020;10:1–16. https://doi.org/10.1038/s41598-020-68350-2.
Ghayor C, Gjoksi B, Siegenthaler B, Weber FE. N-methyl pyrrolidone (NMP) inhibits lipopolysaccharide-induced inflammation by suppressing NF-κB signaling. Inflamm Res. 2015;64:527–36. https://doi.org/10.1007/s00011-015-0833-x.
Acknowledgements
The authors thank the Faculty of Pharmaceutical Sciences, Ubon Ratchathani University, and the School of Engineering and Technology, Walailak University, for support and facilitation. We also thank the Department of Industrial Pharmacy, Faculty of Pharmacy, Silpakorn University, for help and motivation.
Funding
This research was supported with the grant No. SURDI: Postdoctoral/66/5 by Silpakorn University under the postdoctoral fellowship program.
Author information
Authors and Affiliations
Contributions
Conceptualization: T.P.; methodology: E.M.K. and S.S.; validation: K.J. and S.S.; investigation: E.M.K., S.S., and T.P.; writing—original draft preparation: E.M.K., S.S., and T.P.; writing—review and editing: K.J. and T.P.; supervision: K. J. and T.P.; project administration: T.P.; funding acquisition: T.P. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Fluconazole in situ gel oral spray was fabricated using ibuprofen as a gelling agent.
• Higher ibuprofen loading reduced surface tension and retarded solvent exchange.
• The developed system effectively inhibited various Candida species and showed in vitro anti-inflammatory activity.
• Cremophor EL addition increased fluconazole and ibuprofen tissue permeations.
• Fluconazole-loaded ibuprofen in situ gel-based oral spray presents as a promising dosage form for oropharyngeal candidiasis treatment.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khaing, E.M., Senarat, S., Jitrangsri, K. et al. Fluconazole-Loaded Ibuprofen In Situ Gel-Based Oral Spray for Oropharyngeal Candidiasis Treatment. AAPS PharmSciTech 25, 89 (2024). https://doi.org/10.1208/s12249-024-02804-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02804-y