Abstract
Nanoencapsulation is a valid alternative for the oral administration of peptide drugs and proteins, as nanoparticles protect them from proteolytic degradation in the gastrointestinal tract and promote the absorption of these macromolecules. The orofacial antinociceptive effect of frutalin (FTL), through the intraperitoneal route, has already been proven. This study aimed to develop, characterize, and evaluate the orofacial antinociceptive activity of an oral formulation containing FTL in acute and neuropathic preclinical tests. Nanoencapsulated FTL was administered by oral route. The acute nociceptive behavior was induced by administering capsaicin to the upper lip and NaCl to the right cornea. The nociceptive behavior was also induced by formalin injected into the temporomandibular joint. The neuropathic pain model involved infraorbital nerve transection (IONX), which induced mechanical hypersensitivity and was assessed by von Frey stimulation. Trpv1 gene expression was analyzed in the trigeminal ganglion. The analyzed sample did not show any cytotoxicity; 52.2% of the FTL was encapsulated, and the size of the nanocapsule was less than 200 nm, the polydispersion was 0.361, and the zeta potential was − 5.87 and − 12.8 mV, with and without FTL, respectively. Nanoencapsulated FTL administered by oral route had an orofacial antinociceptive effect in acute and neuropathic rodent models. The antinociceptive effect of FTL was prevented by ruthenium red, but not by camphor. FTL reduced Trpv1 gene expression. FTL promotes orofacial antinociception, probably due to the antagonism of TRPV1 channels, and the nanoformulation represents an effective method for the oral administration of this protein.
Highlights
• Nanoformulation for oral protein administration.
• Nanocapsule containing FTL prevents orofacial nociceptive acute and neuropathic pain.
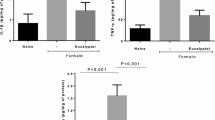
• Frutalin promotes orofacial antinociception behavior antagonism of TRPV1 channels.




Similar content being viewed by others
References
Sarma A, Das MK. Improving the sustainable performance of biopolymers using nanotechnology. Polymer-Plastics Technology and Materials. 2021;60(18):1935–65. https://doi.org/10.1080/25740881.2021.1937645.
Mikusova V, Mikus P. Advances in chitosan-based nanoparticles for drug delivery. International Journal of Molecular Science. 2021;22:1–93. https://doi.org/10.3390/ijms22179652.
Marsili L, Bo MD, Berti F, Toffoli G. Chitosan-based biocompatible copolymers for thermoresponsive drug delivery systems: on the development of a standardization system. Pharmaceutics. 2021;13:1876. https://doi.org/10.3390/pharmacutic13111876.
Zhao D, Yu S, Sun B, Gao S, Guo S, Zhao K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers. 2018;10:1–17. https://doi.org/10.3390/polym10040462.
Hargreaves KM. HHS Public Access, Congress Orofacial Pain. 2011; 3 Suppl 152:25–32. https://doi.org/10.1016/j.pain.2010.12.024.
Romero-Reyes M, Uyanik JM. Orofacial pain management: current perspectives. J Pain Res. 2014; 7:99–115. https://doi.org/10.2147/JPR.S37593.
Shueb SS, Nixdorf DR, John MT, Alonso BF, Durham J. What is the impact of acute and chronic orofacial pain on quality of life? J Dent. 2015;43:1203–10. https://doi.org/10.1016/j.jdent.2015.06.001.
Damasceno MBMV, Melo Junior JMA, Santos SAAR, Melo LTM, Leite LHI., Vieira-Neto AE, Moreira RA, Monteiro-Moreira ACO, Campos AR. Frutalin reduces acute and neuropathic nociceptive behaviours in rodent models of orofacial pain. Chem Biol Interact. 2016; 256:9–15. https://doi.org/10.1016/j.cbi.2016.06.016.
Jagtap UB, Bapat VA. Artocarpus: a review of its traditional uses, phytochemistry and pharmacology. J Ethanopharmacol. 2010;129(2):142–66. https://doi.org/10.1016/j.jep.2010.03.031.
Moreira RA, Castelo-Branco CC, Monteiro-Moreira ACO, Tavares RO, Beltramini LM. Isolation and partial characterization of a lectin from Artocarpus incisa L. Seeds Phytochemistry. 1998;47:1183–8. https://doi.org/10.1016/s0031-9422(97)00753-x.
Zhu Q, Chen Z, Paul PK, Lu Y, Wu W, Qi J. Oral delivery of proteins and peptides: challenges, status quo and future perspectives. 2021; 21(11):2416–2448. https://doi.org/10.1016/j.apsb.2021.04.001.
Haddadzadegan S, Dorkoosh F, Schnurch AB. Oral delivery of therapeutic peptides and proteins: technology landscape of lipid-based nanocarriers. Advanced Drug dDelivery Reviews. 2022;182:1–26.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):68085. https://doi.org/10.1038/227680a0.
Signini R, Sérgio F, Campana SP. Purificação e caracterização de quitosana comercial. Polímeros: Ciênc Tecnol. 1998; 8:63–68. https://doi.org/10.1590/S0104-14281998000400009.
Soares KSR, Fonseca JLC, Bitencourt MAO, Santos KSCR, Silva-Junior AA, Fernandes-Pedrosa MF. Serum production against Tityus serrulatus scorpion venom using cross-linked chitosan nanoparticles as immunoadjuvant. Toxicon. 2012;60:1349–54. https://doi.org/10.1016/j.toxicon.2012.09.010.
Fernandes-Pedrosa MF, Azevedo I.LMJ, Gonçalves-de-Andrade RM, Van Den Berg CW, Ramos CRR, Ho PL, Tambourgi DV. Molecular cloning and expression of a functional dermonecrotic and haemolytic fator from Loxosceles laeta venon. Biochem Biophys Res Commun. 2002; 298(5):638–645. https://doi.org/10.1016/S0006-291X(02)02521-4.
Lee KH, Khan FN, Cosby L, Yang G, Winter JO. Polyme concentration maximizes encapsulation efficiency in electrohydrodynamic mixing nanoprecipitation. 2021; 3:1-14. https://doi.org/10.3389/fnano.2021.719710.
Sawtarie N, Cai Y, Lapitsky Y. Preparation of chitosan/tripolyphosphate nanoparticles with highly tunable size and low polydispersity. Colloids Surf B Biointerfaces. 2017;157:110–7. https://doi.org/10.1016/j.colsurfb.2017.05.055.
Youm I, Yang XY, Murowchick JB, Youan BBC. Encapsulation of docetaxel in oily core polyester nanocapsules intended for breast cancer therapy. Nanoscale Res Lett. 2011; 630:1–12. https://doi.org/10.1186/1556-276X-6-630.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4.
Fadeyi SA, Fadeyi OO, Adejumos AA, Okoro C, Myles EL. In vitro anticancer screening of 24 locally used Nigerian medicinal plants. BMC Complement Altern Med. 2013;13:79–87. https://doi.org/10.1186/1472-6882-13-79.
Pelisser T, Pajot J, Dallel R. The orofacial capsaicin test in rats: effects of different capsaicin concentrations and morphine. Pain. 2002;96:81–7. https://doi.org/10.1016/s0304-3959(01)00432-8.
Melo Júnior JMA, Damasceno MBMV, Santos SAAR, Barbosa TM, Araújo JRC, Vieira-Neto AE, Wong DV, Lima-Júnior RC, Campos AR. Acute and neuropathic orofacial antinociceptive effect of eucalyptol. Inflammopharmacology. 2017;25(2):247–54. https://doi.org/10.1007/s10787-017-0324-5.
Farazifard R, Safarpour F, Sheibani V, Javan M. Eye wi** test: a sensitive animal model for acute trigeminal pain studies. Brain Res Protoc. 2005;16:44–9. https://doi.org/10.1016/j.brainresprot.2005.10.003.
Souza CÁPB, de Oliveira BA, Santos SAAR, Batista FLA, Andrade FRN, Neto EJR, Melo-Júnior JMA, Mendes FRS, Barroso LKV, Canuto KM, Magalhães FEA, Silva ARA, Farias WRL, Campos AR. Orofacial antinociceptive effect of sulphated polysaccharide from the marine algae Hypnea pseudomusciformis in rodents. Inflammopharmacology. 2019;27:261–9. https://doi.org/10.1007/s10787-018-0454-4.
Gameiro GH, Andrade AS, de Castro M, Pereira LF, Tambeli CH, Veiga MCFA. The effects of restraint stress on nociceptive responses induced by formalin injected in rats TMJ. Pharmacol Biochem Behav. 2005;82(2):338–44. https://doi.org/10.1016/j.pbb.2005.09.003.
Roveroni RC, Parada CA, Veiga MCFA, Tambeli CH. Development of a behavioral model of TMJ pain in rats: the formalin test. Pain. 2001;94:185–91. https://doi.org/10.1016/s0304-3959(01)00357-8.
Magalhães FEA, Batista FLA, Serpa OF, Moura LFWG, Lima MCL, Silva ARA, Guedes MIF, Santos SAAR, Oliveira BA, Nogueira AB, Barbosa TM, Holanda DKR, Damasceno MBMV, Melo-Júnior JMA, Barroso LKV, Campos AR. Orofacial antinociceptive effect of Mimosa tenuiflora (Willd.) Poiret. Biomedicine & Pharmacotherapy. 2018; 97;1575–1585. https://doi.org/10.1016/j.biopha.2017.11.001.
Saito K, Hitomi S, Suzuki I, Masuda Y, Kitagawa J, Tsuboi Y, Kondo M, Sessle BJ, Iwata K. Modulation of trigeminal spinal subnucleus caudalis neuronal activity following regeneration of transected inferior alveolar nerve in rats. J Neurophysiol. 2008;99:2251–63. https://doi.org/10.1152/jn.00794.2007.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method. Methods. 2001;25:402–8. https://doi.org/10.1006/meth.2001.126.
Nomura ECO, Rodrigues MRA, da Silva CF, Hamm LA, Nascimento AM, de Souza LM, Cipriani TR, Baggio CH, Werner MFP. Antinociceptive effects of ethanolic extract from the flowers of Acmella oleracea (L.) R.K. Jansen in mice. J Ethnopharmacol. 2013; 50(2):583–589. https://doi.org/10.1016/j.jep.2013.09.007.
Raval N, Maheshwari R, Kalyane D, Youngren-Ortiz SR, Chougule MB, Tekade RK. Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. Basic Fundamentals of Drug Delivery. 2019; 369–400. https://doi.org/10.1016/B978-0-12-817909-3.00010-8.
Streck L, Sarmento VHV, Menezes RPRPB, Fernandes-Pedrosa MF, Martins AMC, Silva-Júnior AA. Tailoring microstructural, drug release properties, and antichagasic efficacy of biocompatible oil-in-water benznidazole-loaded nanoemulsions. Int. J. Pharm. 2018; 555:36–48. https://doi.org/10.1016/j.ijpharm.2018.11.041.
Barros ARC, Damasceno MBMV, Santos SAAR, Melo-Junior JMA, Magalhães FEA, Vieira-Neto AE, Moreira RA, Monteiro-Moreira ACO. TRPV1 mediates the orofacial antinociceptive effect of frutalin: in vivo and in silico Studies. FASEB J. 2017; 31(1):812.6. https://doi.org/10.1096/fasebj.31.1_supplement.812.6.
Ribeiro EF, Barros-Alexandrino TT, Assisd OBG, Juniore AC, Quilesb A, Hernandob I, Nicolettia VR. Chitosan and crosslinked chitosan nanoparticles: synthesis, characterization and their role as Pickering emulsifiers. 2020; 250:1-20. https://doi.org/10.1016/j.carbpol.2020.116878.
Selvamani V. Characterization and biology of nanomaterials for drug delivery. Stability studies on nanomaterials used in Drugs. Elsevier Inc. 2019. p. 425–444.
Samimi S, Maghsoudnia N, Eftekhari RB, Dorkoosh F. Characterization and biology of nanomaterials for drug delivery: nanoscience and nanotechnology in drug delivery micro and nano technologies. Lipid-Based Nanoparticles for Drug Delivery Systems. 2019. p. 47–76.
Funding
The present study was supported by CAPES (#053/2014), CNPq (#302319/2019–0), FUNCAP (#BMD-0124–00063.01.00/17), and Edson Queiroz Foundation (#49/2019).
Author information
Authors and Affiliations
Contributions
Marina de Barros Mamede Vidal Damasceno: conceptualization; investigation; writing, original draft preparation. Sacha Aubrey Alves Rodrigues Santos: investigation; writing, original draft preparation; editing. João Ronielly Campêlo Araújo: investigation. Lana Karine Vasconcelos Barroso: investigation. Samara Casemiro Benevides: investigation. Francisco Ernani Alves Magalhães: investigation. Kaio César Simiano Tavares: investigation. Renato de Azevedo Moreira: investigation. Ana Cristina de Oliveira Monteiro-Moreira: investigation. Angelo Roncalli Alves e Silva: conceptualization; methodology; writing, original draft preparation; editing. Adriana Rolim Campos: conceptualization; methodology; writing, original draft preparation; editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Barros Mamede Vidal Damasceno, M., Santos, S.A.A.R., Araújo, J.R.C. et al. Development of a Nanoformulation for Oral Protein Administration: Characterization and Preclinical Orofacial Antinociceptive Effect. AAPS PharmSciTech 23, 239 (2022). https://doi.org/10.1208/s12249-022-02396-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02396-5