Abstract
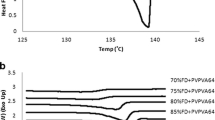
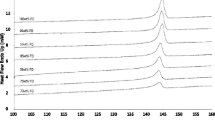
Amorphous solid dispersion (ASD) has been well known as a potential strategy to improve the bioavailability and dissolution performance of poorly water-soluble drugs. The primary concern of this approach is the long-term stability of the amorphous drug in the solid dispersion. Accurate prediction and detection of the solubility and miscibility of drug in polymeric binary system will be a milestone to the development of ASDs. In this investigation, a method based on Flory–Huggins (F–H) theory was proposed to predict and calculate the solubility and miscibility of the drug in polymeric matrix and construct the phase diagram to identify the relevance between drug loading and temperature for ASDs development. Indomethacin (Indo) was chosen as the model drug, and polyvinyl pyrrolidone vinyl acetate (Kollidon® VA 64) was used as a polymeric carrier for the ASD systems. Physical mixtures were prepared with different drug loadings (10 to 90%) and analyzed by differential scanning calorimetry (DSC). The interaction parameter χ was calculated for physical mixtures by the melting point depression and solubility parameter contribution methods. The phase diagram was constructed to investigate the impact of other parameters like drug loading, processing temperature, and Gibbs free energy of mixing (ΔGmix). For further validation, formulations were developed using HME to verify the accuracy of the phase diagram and to guide in the hot-melt extrusion (HME) process design space and optimization.








Similar content being viewed by others
Abbreviations
- ASD:
-
Amorphous solid dispersion
- HME:
-
Hot melt extrusion
- Indo:
-
Indomethacin
- PVP VA 64:
-
Polyvinyl pyrrolidone vinyl acetate
- Kollidon® VA 64:
-
Polyvinyl pyrrolidone vinyl acetate
- F–H theory:
-
Flory-Huggins theory
- G-T equation:
-
Gordon-Taylor equation
- BCS:
-
Biopharmaceutical classification system
- DSC:
-
Differential scanning calorimetry
- TGA:
-
Thermogravimetric analysis
- Eq:
-
Equation
- χ :
-
Interaction parameter
- T g :
-
Glass transition temperature
- T g mix :
-
ASDs glass transition temperature
- T g 1 :
-
Drug glass transition temperature
- T g 2 :
-
Polymer glass transition temperature
- w 1 :
-
Drug weight fraction
- w 2 :
-
Polymer weight fraction
- φ :
-
Drug volume fraction
- φ polymer :
-
Polymer volume fraction
- 1-φ :
-
Polymer volume fraction
- ρ 1 :
-
Drug density
- ρ 2 :
-
Polymer density
- K :
-
Adjustable fitting parameter
- T m :
-
Melting point temperature
- ΔG mix :
-
Gibbs free energy of mixing
- ΔH mix :
-
The change in enthalpy
- ΔS mix :
-
The change in entropy
- δ t :
-
Solubility parameter
- δ d :
-
Components of disperse forces
- δ p :
-
Components of polar group forces
- δ h :
-
Components of hydrogen bond energy
- F di :
-
Group contribution to the disperse forces,
- E hi :
-
Group contribution to hydrogen bonding energy
- F pi :
-
Plane symmetry factor of polar groups
- V 0 :
-
Group contributions to the molar volume
- R 2 :
-
R-Squared
- GFA:
-
Glass forming abilities
- MPD:
-
Melting point depression
- UCST:
-
Upper critical solution temperature
- T min :
-
Minimum processing temperature
- GI fluids:
-
Gastrointestinal fluids
- PM:
-
Physical mixture
- T deg :
-
Degradation temperature
- XRD:
-
X-ray diffraction
- PXRD:
-
Powder X-ray diffraction
- θ :
-
Theta
- RH :
-
Relative humidity
References
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50(1):47–60. https://doi.org/10.1016/s0939-6411(00)00076-x.
Repka MA, Bandari S, Kallakunta VR, Vo AQ, McFall H, Pimparade MB, Bhagurkar AM. Melt extrusion with poorly soluble drugs–an integrated review. Int J Pharm. 2018;535(1–2):68–85. https://doi.org/10.1016/j.ijpharm.2017.10.056.
Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48(1):27–42. https://doi.org/10.1016/s0169-409x(01)00098-9.
Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discovery Today. 2007;12(23–24):1068–75. https://doi.org/10.1016/j.drudis.2007.09.005.
Hancock BC, Parks M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm Res. 2000;17(4):397–404. https://doi.org/10.1023/a:1007516718048.
Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009;98(8):2549–72. https://doi.org/10.1002/jps.21650.
Abu-Diak OA, Jones DS, Andrews GP. An investigation into the dissolution properties of celecoxib melt extrudates: understanding the role of polymer type and concentration in stabilizing supersaturated drug concentrations. Mol Pharm. 2011;8(4):1362–71. https://doi.org/10.1021/mp200157b.
Serajuddin AT. Solid dispersion of poorly water - soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999;88(10):1058–66. https://doi.org/10.1021/js980403l.
Shanbhag A, Rabel S, Nauka E, Casadevall G, Shivanand P, Eichenbaum G, Mansky P. Method for screening of solid dispersion formulations of low-solubility compounds—miniaturization and automation of solvent casting and dissolution testing. Int J Pharm. 2008;351(1–2):209–18. https://doi.org/10.1016/j.ijpharm.2007.09.042.
Zheng W, Jain A, Papoutsakis D, Dannenfelser RM, Panicucci R, Garad S. Selection of oral bioavailability enhancing formulations during drug discovery. Drug Dev Ind Pharm. 2012;38(2):235–47. https://doi.org/10.3109/03639045.2011.602406.
Marsac PJ, Shamblin SL, Taylor LS. Theoretical and practical approaches for prediction of drug–polymer miscibility and solubility. Pharm Res. 2006;23(10):2417–26. https://doi.org/10.1007/s11095-006-9063-9.
Abu-Diak OA, Jones DS, Andrews GP. Understanding the performance of melt-extruded poly (ethylene oxide)–bicalutamide solid dispersions: characterisation of microstructural properties using thermal, spectroscopic and drug release methods. J Pharm Sci. 2012;101(1):200–13. https://doi.org/10.1002/jps.22748.
Gupta J, Nunes C, Vyas S, Jonnalagadda S. Prediction of solubility parameters and miscibility of pharmaceutical compounds by molecular dynamics simulations. J Phys Chem B. 2011;115(9):2014–23. https://doi.org/10.1021/jp108540n.
Marsac PJ, Li T, Taylor LS. Estimation of drug–polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm Res. 2009;26(1):139–51. https://doi.org/10.1007/s11095-008-9721-1.
Paudel A, Van Humbeeck J, Van den Mooter G. Theoretical and experimental investigation on the solid solubility and miscibility of naproxen in poly (vinylpyrrolidone). Mol Pharm. 2010;7(4):1133–48. https://doi.org/10.1021/mp100013p.
Tao J, Sun Y, Zhang GG, Yu L. Solubility of small-molecule crystals in polymers: D-mannitol in PVP, indomethacin in PVP/VA, and nifedipine in PVP/VA. Pharm Res. 2009;26(4):855–64. https://doi.org/10.1007/s11095-008-9784-z.
Sun YE, Tao J, Zhang GG, Yu L. Solubilities of crystalline drugs in polymers: an improved analytical method and comparison of solubilities of indomethacin and nifedipine in PVP, PVP/VA, and PVAc. J Pharm Sci. 2010;99(9):4023–31. https://doi.org/10.1002/jps.22251.
Caron V, Tajber L, Corrigan OI, Healy AM. A comparison of spray drying and milling in the production of amorphous dispersions of sulfathiazole/polyvinylpyrrolidone and sulfadimidine/polyvinylpyrrolidone. Mol Pharm. 2011;8(2):532–42. https://doi.org/10.1021/mp1003674.
Rubinstein M. Polymer physics—The ugly duckling story: will polymer physics ever become a part of “proper” physics? J Polym Sci, Part B: Polym Phys. 2010;48(24):2548–51. https://doi.org/10.1002/polb.22135.
Lin D, Huang Y. A thermal analysis method to predict the complete phase diagram of drug–polymer solid dispersions. Int J Pharm. 2010;399(1–2):109–15. https://doi.org/10.1016/j.ijpharm.2010.08.013.
Hoei Y, Yamaura K, Matsuzawa S. A lattice treatment of crystalline solvent-amorphous polymer mixtures on melting point depression. J Phys Chem. 1992;96(26):10584–6. https://doi.org/10.1021/j100205a002.
Silva MA, De Paoli MA, Felisberti MI. Flory-Huggins interaction parameter of poly (ethylene oxide)/poly (epichlorohydrin) and poly (ethylene oxide)/poly (epichlorohydrin-co-ethylene oxide) blends. Polymer. 1998;39(12):2551–6. https://doi.org/10.1016/S0032-3861(97)00574-0.
Baird JA, Van Eerdenbrugh B, Taylor LS. A classification system to assess the crystallization tendency of organic molecules from undercooled melts. J Pharm Sci. 2010;99(9):3787–806. https://doi.org/10.1002/jps.22197.
Blaabjerg LI, Lindenberg E, Löbmann K, Grohganz H, Rades T. Glass forming ability of amorphous drugs investigated by continuous cooling and isothermal transformation. Mol Pharm. 2016;13(9):3318–25. https://doi.org/10.1021/acs.molpharmaceut.6b00650.
Barton AF. CRC handbook of solubility parameters and other cohesion parameters. Routledge; 2017.
Bhugra C, Pikal MJ. Role of thermodynamic, molecular, and kinetic factors in crystallization from the amorphous state. J Pharm Sci. 2008;97(4):1329–49. https://doi.org/10.1002/jps.21138.
Li S, Tian Y, Jones DS, Andrews GP. Optimising drug solubilisation in amorphous polymer dispersions: rational selection of hot-melt extrusion processing parameters. AAPS PharmSciTech. 2016;17(1):200–13. https://doi.org/10.1208/s12249-015-0450-6.
Newman A, Knipp G, Zografi G. Assessing the performance of amorphous solid dispersions. J Pharm Sci. 2012;101(4):1355–77. https://doi.org/10.1002/jps.23031.
Moseson DE, Taylor LS. The application of temperature-composition phase diagrams for hot melt extrusion processing of amorphous solid dispersions to prevent residual crystallinity. Int J Pharm. 2018;553(1–2):454–66. https://doi.org/10.1016/j.ijpharm.2018.10.055.
Forster A, Hempenstall J, Tucker I, Rades T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int J Pharm. 2001;226(1–2):147–61. https://doi.org/10.1016/S0378-5173(01)00801-8.
Just S, Sievert F, Thommes M, Breitkreutz J. Improved group contribution parameter set for the application of solubility parameters to melt extrusion. Eur J Pharm Biopharm. 2013;85(3):1191–9. https://doi.org/10.1016/j.ejpb.2013.04.006.
Acknowledgements
Authors gratefully acknowledge Rasha El-Kanayati and Joshua Daniel Theus, who kindly reviewed the earlier version of this manuscript and provided valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, Z., Xu, P., Ashour, E.A. et al. Prediction and Construction of Drug-Polymer Binary System Thermodynamic Phase Diagram in Amorphous Solid Dispersions (ASDs). AAPS PharmSciTech 23, 169 (2022). https://doi.org/10.1208/s12249-022-02319-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02319-4